Experiment 7A: Synthesis of Aspirin and Oil of Wintergreen
advertisement

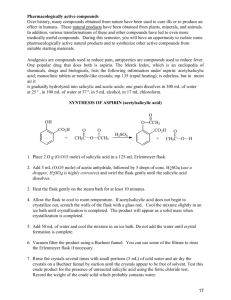
Synthesis of Aspirin and Oil of Wintergreen CHEM 131 Experiment 7 Experiment 7A: Synthesis of Aspirin and Oil of Wintergreen In Experiment 7A, you will engage in the process of organic chemical synthesis by producing two important esters. First, acting as a “flavor chemist,” you will synthesize methyl salicylate (oil of wintergreen). Next, acting as a pharmaceutical chemist, you will synthesize acetylsalicylic acid (aspirin) and qualitatively test its purity. In Experiment 7B, you will perform quantitative analyses of aspirin using skills that you have learned in earlier experiments. BACKGROUND Esters O Esters comprise a class of organic compounds that contain the functional group R C O R' , where R and R’ represent any carbon chain. Esters usually have pleasant, fruitlike odors and are in fact responsible for the flavors and fragrances of many fruits and flowers (as well as for the subtle flavors and aromas ascribed to fine wines by connoisseurs). Some common examples are octyl acetate (oranges), ethyl butyrate (pineapples), methyl butyrate (apples), and isobutyl formate (raspberries). Another important ester is acetylsalicylic acid, better known as aspirin. Esters can be prepared by reacting the functional groups present on alcohols and carboxylic acids in what is known as an esterification reaction. As shown below, this reaction links two different carbon chains together (the R chain from the carboxylic acid and the R’ chain from the alcohol), and produces a water molecule as a byproduct. O R C O H (1) carboxylic acid O + H O R' R C O R' alcohol + H2O ester Any type of reaction in which two molecules are linked together by the removal of a small molecule such as water, is known as a condensation reaction. Thus, esterification is one type of condensation reaction. Organic chemists often use condensation reactions in order to synthesize larger, more complex molecules from smaller, simpler molecules. History of Aspirin For centuries, many people chewed on bits of willow bark to relieve pain. Hippocrates prescribed this remedy around 400 B.C. In 1860, a chemist extracted salicylic acid from willow bark, and found that this was the compound that gave willow bark its pain-relieving properties. Although salicylic acid worked well as a pain reliever, it had some serious drawbacks. Salicylic acid irritates the mouth and esophagus, and causes bleeding in the stomach. As shown below, salicylic acid has a phenol group and a carboxylic acid group. Both of these groups are acidic in character (think about how you could predict this from the molecule’s structure), and this is one of the primary reasons for its harmful characteristics. In 1893, a scientist at the Bayer Company in Germany converted the phenol group in salicylic acid to an ester. This reaction produced acetylsalicylic acid, or aspirin. CHEM 131 Experiment 7A 1 Synthesis of Aspirin and Oil of Wintergreen H H H C C C C O C C H carboxylic acid group C OH H OH phenol group H C C C C H H Salicylic acid O C C O C OH ester group O C CH3 Aspirin (acetylsalicylic acid) Aspirin turned out to be as good a pain-reliever as salicylic acid, or even better. Because aspirin is not converted back to salicylic acid until it reaches the small intestine, aspirin does not have the same drawbacks. Aspirin is now the most widely used pain-reliever in the world. In addition to its pain-relieving properties, aspirin also reduces fever and swelling. It works by inhibiting the formation of prostaglandins, acids that cause inflammation and pain at the site of an injury. Aspirin is one of the safest drugs known, and it has few side effects. However, aspirin does have some side effects. It can prevent synthesis of stomach mucus, leading to ulcer formation on the unprotected stomach walls. Commercial aspirin products sometimes contain buffering agents that reduce the irritation caused by aspirin. Aspirin also prevents blood from clotting. This side effect is also a benefit. Thousands of patients at risk take small daily doses of aspirin to prevent strokes and heart attacks. The Synthesis Reaction Salicylic acid has two functional groups—a phenol group and a carboxylic acid group. When salicylic acid is converted into aspirin, its phenol group reacts to form the ester. The remainder of the molecule would thus be represented by the symbol R’ in the esterification reaction equation (1) shown on page 1. We can see that the second carbon chain of the ester (the R group) is a methyl (-CH3) group. Consequently, in order to convert salicylic acid into aspirin, we would need to react it with the carboxylic acid, CH3COOH (acetic acid). H H C C H C C O C C carboxylic acid group C OH H OH R’ + H3PO4 catalyst OH phenol group H C O H CH3 C C H O C C C H R C C O O C R’ OH + H2O ester group CH3 R Salicylic acid Acetic acid Aspirin (acetylsalicylic acid) While it is theoretically possible to synthesize aspirin by reacting salicylic acid with acetic acid, this reaction is not as efficient (in terms of both speed and yield of product) as using acetic anhydride. An anhydride is two carboxylic acids that are joined through a condensation reaction. This molecule is less stable than an ordinary carboxylic acid, and thus reacts with the salicylic acid more readily. We will use acetic anhydride, rather than acetic acid, in order to synthesize aspirin. As shown in the following reaction equation, this will produce acetic acid, rather than water, as the byproduct of the condensation reaction. CHEM 131 Experiment 7A 2 Synthesis of Aspirin and Oil of Wintergreen H H H C C C C O C C H carboxylic acid group C OH O C OH + O phenol group C H3PO4 catalyst H H CH3 Acetic anhydride C C C C H O H Salicylic acid CH3 O C C O C O C O OH + ester group CH3 C OH CH3 Aspirin (acetylsalicylic acid) Acetic acid Salicylic acid can also be converted into a different ester, methyl salicylate (oil of wintergreen). In this esterification reaction (shown near the bottom of p.474 of your textbook), it is the carboxylic acid group of salicylic acid that reacts, in this case with methanol, CH3OH. Impurities in Aspirin Salicylic acid is a common impurity in aspirin. The presence of salicylic acid is undesirable, because it causes more irritation and stomach bleeding than aspirin does. The FDA allows a maximum of 0.15% salicylic acid in commercial aspirin tablets. You can test for salicylic acid by adding 1% FeCl3 solution to aspirin samples. FeCl3 reacts with most compounds containing phenol groups to give a purple product. Aspirin does not have a phenol group. (No matter how pure your aspirin appears, you should not eat it!) There are two ways that salicylic acid impurities can get into aspirin samples. First, there might be some left over from the reaction used to make the aspirin. Normally, pharmaceutical companies remove un-reacted salicylic acid from aspirin before making it into tablets. Second, old aspirin can hydrolyze (break down) into salicylic acid and acetic acid (vinegar). (This hydrolysis reaction will be explored further in Experiment 7B.) If you have an old bottle of aspirin that smells like vinegar, you should throw it away. Prelab Questions for Experiment 7A For help with Question 1, see p.472 and Tables 10.7, 10.10, and 10.11 of your textbook. 1. There are two esters with chemical formula C3H6O2. (a) Write their structural formulas and name them. (b) Write a chemical equation showing how each could be prepared by reacting appropriate alcohols and carboxylic acids. 2. What is the theoretical yield of aspirin (180 g mol-1) from the reaction of 2.0 g salicylic acid (138 g mol-1) with 5.0 mL of acetic anhydride (d = 1.082 g/mL, 102 g mol-1)? Experiment 7A: Synthesis of Aspirin and Oil of Wintergreen Procedure A. Synthesis of Methyl Salicylate 1. Place approximately 1g of salicylic acid and 5 mL of methanol in a large test tube, and add 5 drops of concentrated phosphoric acid. 2. Place the test tube in a 70ºC hot water bath (use a 400 mL beaker) for about 15 min. (While you are waiting, you can begin part B.) 3. Stir the mixture, note the odor and record this observation in your notebook. CHEM 131 Experiment 7A 3 Synthesis of Aspirin and Oil of Wintergreen B. Synthesis of Aspirin 1. Weigh out 2.0 grams of salicylic acid and place it in a clean and dry 125-mL Erlenmeyer flask. 2. In the fume hood, measure out 5.0 mL of acetic anhydride using a graduated cylinder. Slowly add the acetic anhydride to the salicylic acid in the flask. 3. Add 10 drops of concentrated phosphoric acid to the flask to catalyze the reaction. Gently stir with a glass stirring rod. Now you may take your flask back to your lab bench. 4. Place the flask in the boiling water bath. Stir constantly until all the solid dissolves. Continue heating for about 15 minutes. 5. Remove the flask from the hot water and let it cool down to room temperature. 6. In the fume hood, SLOWLY add 20 drops of distilled water to the reaction mixture while swirling the contents of the flask. The water destroys any remaining acetic anhydride, by turning it into acetic acid vapors. When the reaction with water is complete, you may take your flask back to your lab bench. 7. Gradually add 25 mL of cold distilled water to the flask while swirling. Place the flask in an ice bath for 10 minutes. If no crystals form, scratch the inside of the flask with a glass stirring rod. 8. Set up a filter flask with a Buchner funnel and filter paper for suction filtration, as demonstrated by your instructor. Turn on the water to start the suction, and wet the filter paper with distilled water. 9. Pour the aspirin crystals and water into the funnel. Use a spatula and a few 5-mL portions of ice-cold distilled water to rinse any remaining crystals out of the flask. Pour a final 5-mL portion of ice-cold distilled water over the crystals to be sure you have removed any remaining acetic acid. 10. Spread the aspirin crystals out on the filter paper and draw air through the funnel. Try to remove as much water as possible from the aspirin crystals. 11. Turn off the water. Use a spatula to lift the filter paper and aspirin crystals from the funnel to a paper towel. Place a small sample of your aspirin (the end of a microspatula) in a labeled test tube and save it for part C. Weigh the remaining portion of your aspirin. C. Testing for Purity 1. You will be testing 3 different materials for the presence of phenols, as indicated below. 2. Test tube 1 should contain a few crystals (the small amount that will fit on the tip of a spatula) of salicylic acid. 3. Test tube 2 should contain aspirin (from part B). 4. Test tube 3 should contain 2 mL 0.15% salicylic acid solution. This solution has the maximum amount of salicylic acid allowed by the FDA. 5. Add 2 mL of distilled water to each of test tubes 1 and 2. Shake to mix. 6. Add 5 drops of 1% FeCl3 solution to each test tube. Record your observations. CHEM 131 Experiment 7A 4