Infrared Spectroscopy
advertisement

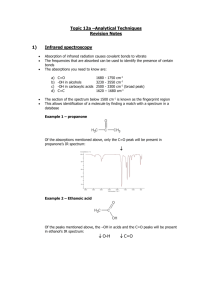
1 Infrared Spectroscopy The Electromagnetic Spectrum History: Discovery of Infrared Light Molecular Spectra Quantitative Analysis Fourier-Transform Spectrometry Laser Diode Spectrometry Remote Sensing 2 The Electromagnetic Spectrum http://www.lbl.gov 3 Spectroscopy • Spectral nature of light present in the rainbow, but beyond ability of early humans to explain it • Newton (1666): white light from the sun could be dispersed into series of colors • Newton introduced the word spectrum • Instrument: aperture, lens, glass prism and a screen to display the resulting spectrum 4 History • 1666 Newton observes spectrum obtained from a prism • 1678 Huygens proposes wave theory of light • 1729 Pierre Bouger notices attenuation of light as it passes through successive thicknesses of glass • 1752 Thomas Melville writes of yellow light in flame when sea salt mixed with alcohol • 1760 Johann Lambert gives mathematical formula to Bouger • 1776 Alessandro Volta notes different colors when using different sparking materials • 1800 F.W. Herschel discovers infrared light http://www.cofc.edu/~deavorj/521/spechist.html http://web.mit.edu/spectroscopy/history/history-classical.html 5 History: Discovery of Infrared Light • Sir Friedrich Wilhelm (William) Herschel (1738-1822) • Discovery of the planet Uranus in 1781 • Discovery of infrared light (1800) Prism Thermometers 6 History (Continued) • 1801 J.W. Ritter discovers ultraviolet light • 1802 Thomas Young demonstrates wave nature of light by using a transmission grating • 1817 Josef Fraunhofer maps details of absorption lines in solar spectrum • 1826 W.H.F. Talbot notices different salts yield different colored flames • 1852 August Beer shows logarithmic relation of Lambert • 1859 G. Kirchoff and R.W. Bunsen are the first to state that elements have both characteristic absorption and emission spectra • 1891 A. A. Michelson invents interferometer (Nobel Prize in Physics 1907) http://www.cofc.edu/~deavorj/521/spechist.html http://web.mit.edu/spectroscopy/history/history-classical.html 7 Definitions • Wavenumber σ = ν/c , ν: frequency, c: velocity of light (vacuum) Unit of wavenumber ([σ] = 1 Kayser = 1 cm-1) is used as frequency unit • Radiance L: Power (radiant flux) Φ per solid angle and area, perpendicular to direction of propagation: L=d2Φ/(dAdΩ cosϑ) • dΩ solid angle, dA area, ϑ angle between direction of propagation and normal vector of dA • Spectral radiance: Lσ = dL/dσ • Irradiance (Intensity): I = Φ/A A: Area 8 Energy Levels of Molecules • Energy is the sum of energy of the electrons, vibrational energy, and rotational energy E = Eel + Evib + Erot • Energy difference between different levels (orders of magnitude) ∆Eel ≈ 10 eV λ ≈ 100 nm σ ≈ 105 cm-1 Ultraviolet (UV) ∆Evib ≈ 0.1 eV λ ≈ 10 µm σ ≈ 1000 cm-1 Infrared (IR) ∆Erot ≈ 10-4 eV λ ≈ 1 cm σ ≈ 1 cm-1 Microwaves 9 Diatomic Molecule: Rotation • Rotation: • Classical mechanics: Z 2 L E= 2I M1 E: Energy L: Angular momentum I : Moment of inertia M2 R J (J + 1)h 2 • Quantum mechanics: E = 2I J: Quantum number of rotation J = 0, 1 … h=h/2π h =6,62607×10-34 J s Planck´s constant 10 Diatomic Molecule: Vibration Z • Nuclei in the field of the electrons ⇒ Attracting Force M1 M2 • Coulomb-force (Nuclei positive) ⇒ Repelling Force R 2 • Potential energy: Energy (De) 1.5 Repelling force 1 0.5 Attracting Force 0 0.5 1 0 1 2 3 Distance R (Ro) 4 5 11 Diatomic Molecule Z • Description of Vibration M1 • M2 Morse-Potential: empirical formula for the potential energy E pot ( R ) D e . e 2. α. R R0 2. e α. R R R0 2 • Approximation for R ≈ R0: harmonic oscillator R0 • Eigenvalues of the energy : E • υ 1 . . hω 2 ν = 0,1.... • Energy levels are equidistant (harmonic oscillator) 1 2 Epot (De) 2 E pot ( R ) D e . α . R ES 0 1 0 1 2 3 Distance R/R Kernabstand R (Ro) 0 4 5 12 Spectra of Molecules • Transitions between energy levels • Vibrational spectra contain rotational fine structure • Selection rules 13 Vibration-Rotation Spectrum Selection rules (harmonic oscillator): ∆J = +- 1 (exception: ∆J = 0, +- 1 if electronic quantum number of angular momentum Λ ≠ 0) ∆ν = +- 1 14 Vibration-Rotation Spectrum of HCl 0.08 Absorption 0.06 0.04 0.02 0 Relation between Energy and frequency of radiation: E’- E’’ = h·f = h·c·σ Absorption 0.02 2600 2800 2900 3000 Wavenumber (cm-1) P-Branch (Q-Branch) R-Branch 3100 3200 0.05 0 2900 Upper state Lower state 2700 2920 2940 2960 2980 3000 3020 Wavenumber (cm-1) h: Planck’s constant, c: velocity of light, σ: Wavenumber 3040 3060 3080 15 Polynuclear Molecules • Calculation of resonance frequencies • Description of positions of nuclei of the molecule by 3N – 6 coordinates, N: number of nuclei (Linear molecule: 3N – 5) • 3N – 6 (5) coupled equations of motion • Calculation of eigenvalues and eigenvectors • Diagonalisation: Decoupling of equations • 3N – 6 (5) normal modes 16 Absorption Lines of CO2 0.8 Absorption 0.6 0.4 0.2 0 620 640 660 680 Wavenumber σ / cm-1 700 720 17 Group Frequencies http://www.cem.msu.edu/~reusch/VirtualText/Spectrpy/InfraRed/infrared.htm 18 Summary: Vibration-Rotation Spectra of Molecules • Allowed energy states of molecules are quantized • In the infrared spectral region, transitions between vibrational states of molecules are observed • Rotational structure of vibrational transition (band) • All molecules except diatomic homonuclear molecules (e.g. N2, O2) have significant characteristic signatures in the infrared spectral region • Property that allows observation by spectroscopy: Dipole moment is (non-constant) function of nuclear positions • Functional groups have specific absorption bands • Molecule with N nuclei has 3N-6 normal modes, i.e. 3N-6 (possible) absorption bands (linear molecule: 3N-5)