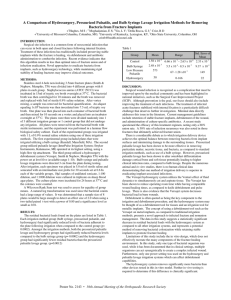
Comparison of Bulb Syringe, Pressurized Pulsatile, and
advertisement

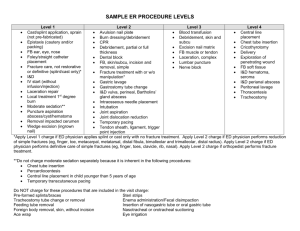
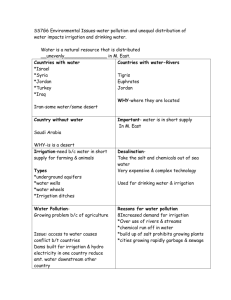
n Feature Article Comparison of Bulb Syringe, Pressurized Pulsatile, and Hydrosurgery Debridement Methods for Removing Bacteria From Fracture Implants Michael S. Hughes, MD; Eric S. Moghadamian, MD; Li-Yan Yin, MD; Gregory J. Della Rocca, MD, PhD; Brett D. Crist, MD abstract Full article available online at Healio.com/Orthopedics. Search: 20120621-19 Surgical-site infection is a common form of noscomial infection that can occur in fractures following internal fixation. Treatment of these infections has traditionally included preserving stable implants via debridement and antibiotic administration while the fracture is healing. Recent evidence indicated that this algorithm results in lessthan-optimal rates of fracture union and infection eradication. The premise for this study is that bacterial removal from fracture implants using the Versajet Hydrosurgery System (Smith & Nephew, Memphis, Tennessee) method is better compared with the bulb syringe and pressurized pulsatile lavage methods. Thirty-two stainless steel, 4-hole, nonlocking, 3.5-mm fracture plates were incubated with Staphylococus aureus and divided into 4 groups. Eight plates in each group underwent irrigation with 1 L of saline using a bulb syringe lavage, pressurized pulsatile lavage, or the Versajet Hydrosurgery System method. Eight plates underwent no irrigation method and served as a control group. The residual bacterial loads following irrigation were quantitatively cultured. Each of the experimental groups had significantly reduced levels of bacteria adherent to the plate following irrigation compared with the control group (P5.0002). Furthermore, the Versajet Hydrosurgery System was most the effective at bacterial removal, followed by the pressurized pulsatile and bulb syringe lavage techniques (P5.0002 to P5.0012, respectively). A B Figure: Photographs prior to (A) and during (B) use of the Versajet Hydrosurgery System (Smith & Nephew, Memphis, Tennessee) on the infected ankle fracture with a sterile radiograph cassette cover placed over the wound to prevent splatter. Novel approaches to eradicate bacteria from implants, such as hydrosurgery technology, while maintaining rigid stability of healing fracture, may improve clinical outcomes. Dr Hughes is from Washington University, St Louis, Drs Della Rocca and Crist are from the Department of Orthopaedic Surgery, University of Missouri, Columbia, Missouri; Dr Moghadamian is from the Department of Orthopaedics, University of Kentucky, Lexington, Kentucky; and Dr Yin is from the Department of Orthopaedics, Ohio State University, Columbus, Ohio. Dr Hughes received a Multi-Purpose Resident Grant from the Mid-America Orthopaedic Association. Drs Moghadamian, Yin, Della Rocca, and Crist have no relevant financial relationships to disclose. Smith & Nephew and Stryker donated equipment necessary for the completion of the study. This investigation was performed at the University of Missouri School of Medicine, Columbia, Missouri. Correspondence should be addressed to: Brett D. Crist, MD, Department of Orthopaedic Surgery, University of Missouri, Columbia, 1 Hospital Dr, Columbia, MO 65212 (cristb@health.missouri.edu). doi: 10.3928/01477447-20120621-19 e1046 ORTHOPEDICS | Healio.com/Orthopedics Debridement Methods for Implants | Hughes et al I n 2006, approximately 43,000 fractures were treated with open reduction and internal fixation (ORIF) in the United States.1 Surgical-site infection occurs at a rate of ,5% after ORIF of closed fractures.2 Acute ORIF of open fractures has decreased infection rates compared with historical methods of delayed stabilization.3 However, the incidence of infection after open fractures is still reported to be as high as 50%.4 The progression of bacterial contamination to the infection of fracture fixation implants is a well documented and and difficult to treat phenomenon.5 The treatment of infected fractures typically includes retaining stable fracture fixation implants, irrigation and debridement procedures, and the administration of culture-directed antibiotics (locally, systemically, or both) to suppress the infection until fracture union occurs.6 This treatment method places priority on the maintenance of fracture stability over the complete eradication of the bacterial load. However, recent studies reported that this treatment method resulted in a 29% to 32% nonunion rate and a 30% to 50% recurrence rate of infection in the fractures that ultimately achieved union.6,7 It is possible that commonly used methods of debridement and irrigation of infected fracture beds with retained implants are not sufficiently effective at reducing bacterial loads. A novel surgical debridement tool, such as the Versajet Hydrosurgery System (Smith & Nephew, Memphis, Tennessee), may be a more effective tool for debridement compared with traditional methods. The Versajet Hydrosurgery System uses the Venturi effect of fluid dynamics, where a highpressure stream of saline is used tangentially to cut tissue while simultaneously creating a local vacuum that facilitates the removal of tissue or foreign debris. The purpose of this ex vivo study was to evaluate the efficacy of the Versajet Hydrosurgery System in removing adherent bacteria from stainless steel fracture plates compared with the traditional meth- JULY 2012 | Volume 35 • Number 7 ods of bulb syringe and pressurized pulsatile lavage. The null hypothesis is that no difference will exist in the amount of residual bacterial loads on incubated fracture plates after irrigation between these 3 techniques. Materials and Methods Staphylococcus aureus, a common pathogen found in approximately 65% of infected orthopedic wounds, was selected for this experiment. Staphylococcus aureus (29213; American Type Culture Collection, Rockville, Maryland) was incubated in 15 mL of tryptic soy broth for 24 hours at 37°C. The broth was then centrifuged for 10 minutes, and the supernatant was discarded. The bacterial pellet was resuspended in 5 mL of 0.9% sodium chloride solution. A 20-µL sample was removed, and the bacterial count was quantified. Multiple flasks of 7.5 mL of tryptic soy broth were then incubated with 1,000,000 bacteria. Thirty-two sterile, stainless-steel, 4-hole, conventional fracture plates (Smith & Nephew) were used in this protocol. One plate was placed into each container of bacterial broth and mixed for 10 seconds on a vortex (Analog Mixer 02-215-365; Fisher Scientific, Waltham, Massachusetts). Plates were then incubated for 24 hours at 37°C. At the conclusion of incubation, all plates were removed from the bacterial broth. Irrigation of the plates was performed in a laminar flow biological safety cabinet (A2; Thermo Scientific, Waltham, Massachusetts). The 32 plates were divided equally into 4 groups. Plates from each of the 3 experimental groups were irrigated with 1 L of sterile 0.9% saline solution: group 1 with a standard bulb syringe (Kendall, Mansfield, Massachusetts), group 2 with a low-pressure pulsatile lavage (InterPulse Irrigation System; Stryker, Kalamazoo, Michigan) operated at its highest setting using a high-flow tip attachment (model 210-14; maximum pressure, 19 psi; maximum flow rate, 1025 mL/min), and group 3 with the Versajet Hydrosurgery System (at power setting 5). A fourth control group of 8 plates was not exposed to irrigation. Bulb syringe and pressurized pulsatile lavage irrigation devices were held 3 cm from the plates during testing. The Versajet Hydrosurgery System requires plate contact for debridement. At the completion of irrigation, each plate was placed in 5.0 mL of sterile saline and sonicated with an intermediate size probe (Artek Sonic Dismembrator, Model 301; Dynatech Laboratories, Chantilly, Virginia) for 30 seconds at 0.6 power. For each group, 10-µL samples of undiluted sonicate, 1:100 sonicate dilution, and 1:1000 sonicate dilution were cultured in triplicate on sheep blood agar plates (Fisher Scientific). In the control group, the 1:100 dilution was replaced with a 1:100,000 dilution on the agar plate because further dilution was required to count the individual colonies (due to the large amount of bacterial growth). The culture plates were incubated for 24 hours at 37°C, and the colonies were counted manually. Statistical analysis was performed using SAS Institute, Inc, version 9 software (Cary, Northo Carolina). The Wilcoxon rank sum test was used to assess equality of group means. A natural log transformation was also used because the bacterial counts exhibited a wide range of variability. A sample size of 8 plates per group was large enough to detect an effect size of 2.0 when using a 2-tailed paired t test with a power of 0.80 and a significance level as small as .01. Results Residual bacterial loads for each treatment group are shown in Table 1. Decreased residual bacterial counts were noted in each group as compared with the control group (P5.0002). Statistically significant differences existed between the means of each of the treatment groups (Table 2). The pressurized pulsatile lavage and Versajet Hydrosurgery System groups had significantly fewer residual bacteria e1047 n Feature Article than the bulb syringe group (P5.0002). The Versajet Hydrosurgery System group had significantly lower bacterial counts than the pulsatile lavage group (P5.0012). Although the irrigation systems with higher pressure removed significantly more bacteria, aerosolization of irrigant (presumably containing bacteria) was noted with use of the higherpressure systems, especially the Versajet Hydrosurgery System. An example of the pattern of contaminated fluid dispersion with the Versajet Hydrosurgery System is shown in Figure 1. Debridement Methods for Implants | Hughes et al Table 1 Residual Bacterial Load on Plate Following Irrigation in Colony-forming Units Group Mean Control 1.58310 Bulb syringe lavage a e1048 SE of Mean 6.66310 to 2.63 x 10 7 8 2.333107 2.483105 5.53104 to 8.53105 9.373104 b 573 67 to 1.283103 149 c 50 0 to 166 22 Low-pressure pulsatile lavage Versajet Hydrosurgery System a Kendall, Mansfield, Massachusetts. b Interpulse Irrigation System; Stryker, Kalamazoo, Michigan. c Smith & Nephew, Memphis, Tennessee. Discussion Surgical wound infection prevention is a high priority for surgeons, patients, and hospital systems. Once infection has occurred, optimizing its treatment is imperative. The purpose of this study was to evaluate the effectiveness of different irrigation and debridement methods on bacterial removal from fracture fixation plates in an ex vivo fashion. This study does not recreate the complex in vivo setting of a fracture stabilized with fracture fixation implants that are colonized with bacteria, such as eradicating bacteria from crevices under the implant or the interface of the screw head with the plate. A paucity of data exists in the literature regarding the effectiveness of current infection management strategies in the setting of healing fractures stabilized with metallic implants. Current management strategies often include the retention of stable fracture implants, debridement of the wound, and adjunctive antibiotic administration. Recent studies have reported suboptimal fracture union rates (range, 68% to 71%) and high rates of recurrent infection (range, 30% to 50%) in fractures that achieved union.6,7 In the arthroplasty literature, debridement and systemic antibiotic administration with the preservation of infected implants has been associated with poor outcomes, with success rates of approximately 31%.8 Instead, staged manage- Range 8 Table 2 Mean Residual Bacterial Loads Statistical Comparison in Colony-forming Units Group Bulb Syringe Lavage Low Pressure Pulsatile Lavage Versajet Hydrosurgery System P 1.58310 2.48310 - - .0002 1.58310 - 573 - .0002 1.58310 - - 50 .0002 - 2.483105 573 - .0002 - 2.483105 - 50 .0002 - - 573 50 .0012 Control 8 8 8 5 a Kendall, Mansfield, Massachusetts. b Interpulse Irrigation System; Stryker, Kalamazoo, Michigan. c Smith & Nephew, Memphis, Tennessee. ment of the infected arthroplasty, consisting of hardware explantation and the administration of locally and systemically delivered antibiotics followed by delayed reimplantation when signs of infection are absent, has resulted in successful outcomes in up to 90% of patients.9 A bacterial wound infection begins with the adhesion of bacteria to a surface, which occurs in 4 distinct stages: nonspecific attachment, specific attachment, in situ multiplication, and release and dissemination.10 Irrigation methods that are used for the treatment of infected wounds must balance the effective removal of bacteria with tissue damage associated with the debridement proce- 1 Figure 1: Photograph of the laboratory setting that was used to aerosolize saline during irrigation of bacteria-contaminated plates with the Versajet Hydrosurgery System (Smith & Nephew, Memphis, Tennessee). ORTHOPEDICS | Healio.com/Orthopedics Debridement Methods for Implants | Hughes et al 2A 2B Figure 2: Photographs prior to (A) and during (B) use of the Versajet Hydrosurgery System (Smith & Nephew, Memphis, Tennessee) the infected ankle fracture with a sterile radiograph cassette cover placed over the wound to prevent splatter. dure. Pressurized pulsatile lavage has been more effective in vitro at removing particulate matter, necrotic tissue, and bacteria from tissue compared with low-pressure irrigation methods, such as the use of a bulb syringe.11,12 However, it may also alter osteoblast differentiation, damage cortical bone and soft tissue, and increase bacterial penetration into tissues, potentially leading to higher infection rates.13-17 Other studies have shown that pressurized pulsatile lavage may not have an adverse effect on fracture callus and the soft tissue that envelopes the surrounding bone.18-20 Pressurized pulsatile lavage has also been reported to propagate bacterial contamination into deeper tissues and distant sites, such as in the medullary canal.21 However, other data indicate that it does not differ from bulb syringe.22 Despite numerous animal and in vitro studies, no human clinical data exist that demonstrate superiority of one method of irrigation over another with regard to eradicating or decreasing the occurrence of infections. The Versajet Hydrosurgery System has the ability to cut and aspirate tissue simultaneously in a plane reportedly as thin as 50 µm. It has reduced operative time without adversely affecting wound healing times when compared with knife debridement and pulsatile lavage.23 Evidence also exists that it decreases bacterial loads in burn wounds.24 Debridement is often quoted as being the key component of the irrigation and debridement procedure, and the Versajet JULY 2012 | Volume 35 • Number 7 Hydrosurgery System may be considered a method of accomplishing both. The use of a hydrosurgery debridement tool on metal implants may represent a novel approach to infected fracture and nonunion management. It allows for the simultaneous removal of local necrotic tissue and bacteria from implants and tissue. This study demonstrates that use of the Versajet Hydrosurgery System is more effective than other tested irrigation methods of bacteria removal from stainless steel implants. It deserves further research investigating its applicability as a debridement tool in the clinical setting. Limitations of this study include its in vitro design, which does not effectively recreate the clinical scenario of infection following fracture fixation. Only onebacteria species (S aureus) was studied, but many surgical wound infections occur from different organisms and may be polymicrobial. Only one power setting was used on the Versajet Hydrosurgery System and the pressurized pulsatile lavage system, and this experiment included no other methods of debridement, such as suction-brush instruments that have been successful in removing contaminants in similarly designed ex vivo studies.25 During this study, the authors noted that splatter and aerosolization of the irrigation fluids with use of the Versajet Hydrosurgery System was substantial. It is possible that redirection of the fluid outside of the vacuum circuit of the Versajet Hydrosurgery System (ie, splatter) occurs when the water stream cannot cut into the surface against which it is apposed (in the current case, the metallic plate). Surgeons are encouraged to consider the use of inexpensive shielding devices, such as sterile radiograph cassette covers, during debridement with the Versajet Hydrosurgery System to reduce hazard to the operative team (Figure 2).26 Conclusion Irrigation and debridement are key components in managing infected fractures with retained implants. Multiple methods of irrigation exist. Of the 3 modalities (ie, bulb syringe lavage, pressurized pulsatile lavage, and Versajet Hydrosurgery System) that were tested in this ex vivo study, the Versajet Hydrosurgery System was the most effective in reducing the residual bacterial colonization of a stainless steel fracture fixation plate. References 1. DeFrances CJ, Lucas CA, Buie VC, Golosinskiy A. 2006 National Hospital Discharge Survey. Natl Health Stat Report. 2008; 30:1-20. 2. Boxma H, Broekhuizen T, Patka P, Oosting H. Randomised controlled trial of singledose antibiotic prophylaxis in surgical treatment of closed fractures: the Dutch Trauma Trial. Lancet. 1996; 347:11331137. 3. Worlock P, Slack R, Harvey L, Mawhinney R. The prevention of infection in open fractures: an experimental study of the effect of fracture stability. Injury. 1994; 25:31-38. 4. Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976; 58:453-458. 5. Schmidt AH, Swiontkowski MF. Pathophysiology of infections after internal fixation of fractures. J Am Acad Orthop Surg. 2000; 8:285-291. 6.Rightmire E, Zurakowski D, Vrahas M. Acute infections after fracture repair: management with hardware in place. Clin Orthop Relat Res. 2008; 466:466-472. 7. Berkes M, Obremskey WT, Scannell B, et al. Maintenance of hardware after early postoperative infection following fracture internal fixation. J Bone Joint Surg Am. 2010; 92:823828. e1049 n Feature Article 8. Marculescu CE, Berbari EF, Hanssen AD, et al. Outcome of prosthetic joint infections treated with debridement and retention of components. Clin Infect Dis. 2006; 42:471478. 9. Kraay MJ, Goldberg VM, Fitzgerald SJ, Salata MJ. Cementless two-staged total hip arthroplasty for deep periprosthetic infection. Clin Orthop Relat Res. 2005; 441:243-249. 10.Gristina AG, Costerton JW. Bacterial ad herence to biomaterials and tissue. The significance of its role in clinical sepsis. J Bone Joint Surg Am. 1985; 67:264-273. 11. Bhandari M, Schemitsch EH, Adili A, Lachowski RJ, Shaughnessy SG. High and low pressure pulsatile lavage of contaminated tibial fractures: an in vitro study of bacterial adherence and bone damage. J Orthop Trauma. 1999; 13:526-533. 12. Moussa FW, Gainor BJ, Anglen JO, Christensen G, Simpson WA. Disinfecting agents for removing adherent bacteria from orthopaedic hardware. Clin Orthop Relat Res. 1996: 255-262. 13. Dirschl DR, Duff GP, Dahners LE, Edin M, Rahn BA, Miclau T. High pressure pulsatile lavage irrigation of intraarticular fractures: effects on fracture healing. J Orthop Trauma. 1998; 12:460-463. e1050 Debridement Methods for Implants | Hughes et al 14. Boyd JI III, Wongworawat MD. High-pressure pulsatile lavage causes soft tissue damage. Clin Orthop Relat Res. 2004:13-17. 15.Hassinger SM, Harding G, Wongworawat MD. High-pressure pulsatile lavage propagates bacteria into soft tissue. Clin Orthop Relat Res. 2005; 439:27-31. 16. Owens BD, White DW, Wenke JC. Com parison of irrigation solutions and devices in a contaminated musculoskeletal wound survival model. J Bone Joint Surg Am. 2009; 91:92-98. 17. Bhandari M, Schemitsch EH. High-pressure irrigation increases adipocyte-like cells at the expense of osteoblasts in vitro. J Bone Joint Surg Br. 2002; 84:1054-1061. 18. Adili A, Bhandari M, Schemitsch EH. The biomechanical effect of high-pressure irrigation on diaphyseal fracture healing in vivo. J Orthop Trauma. 2002; 16:413-417. 19.Caprise PA Jr, Miclau T, Dahners LE, Dirschl DR. High-pressure pulsatile lavage irrigation of contaminated fractures: effects on fracture healing. J Orthop Res. 2002; 20:1205-1209. 20.Di Pasquale DJ, Bhandari M, Tov A, Schemitsch EH. The effect of high and low pressure pulsatile lavage on soft tissue and cortical blood flow: a canine segmental hu- merus fracture model. Arch Orthop Trauma Surg. 2007; 127:879-884. 21. Bhandari M, Adili A, Lachowski RJ. High pressure pulsatile lavage of contaminated human tibiae: an in vitro study. J Orthop Trauma. 1998; 12:479-484. 22. Lee EW, Dirschl DR, Duff G, Dahners LE, Miclau T. High-pressure pulsatile lavage irrigation of fresh intraarticular fractures: effectiveness at removing particulate matter from bone. J Orthop Trauma. 2002; 16:162165. 23. Caputo WJ, Beggs DJ, DeFede JL, Simm L, Dharma H. A prospective randomised controlled clinical trial comparing hydrosurgery debridement with conventional surgical debridement in lower extremity ulcers. Int Wound J. 2008; 5:288-294. 24. Klein MB, Hunter S, Heimbach DM, et al. The Versajet water dissector: a new tool for tangential excision. J Burn Care Rehabil. 2005; 26:483-487. 25. Draeger RW, Dirschl DR, Dahners LE. Debridement of cancellous bone: a comparison of irrigation methods. J Orthop Trauma. 2006; 20:692-698. 26. Greene DL, Akelman E. A technique for reducing splash exposure during pulsatile lavage. J Orthop Trauma. 2004; 18:41-42. ORTHOPEDICS | Healio.com/Orthopedics