Synthesis and Analysis of Banana Oil
advertisement

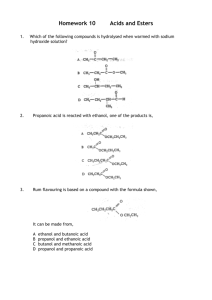
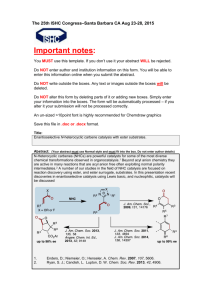
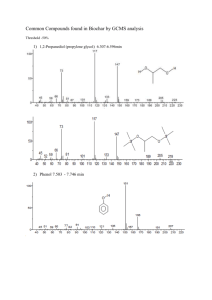
Synthesis and Analysis of Banana Oil Introduction: This experiment will introduce you to several of the techniques used in organic chemistry to prepare pure compounds. The compound must be separated from any other substances used or produced in preparing the compound. When the compound to be prepared is a liquid (as in this experiment), and if it is mixed with another liquid in which it is insoluble, it can be separated from that liquid by using a separatory funnel (a funnel with a stopcock allowing the heavier liquid to be separated from the lighter). The desired liquid compound can be separated from remaining impurities by distillation. In distillation the liquid is boiled, and resulting vapors are passed through a condenser. The resulting liquid is collected. Low boiling components will pass over first so a separation can be made. The compound you will prepare is an ester. Esters are organic compounds formed by the reaction of alcohols with carboxylic acids. Though most carboxylic acids possess a sour taste and smell, esters impart a pleasant odor and are often responsible for the flavor and scent of fruits and flowers. The following general reaction illustrates the preparation of an ester from an acid and alcohol (with sulfuric acid as a catalyst). O R C OH carboxylic acid + R' OH H2SO4 O R alcohol C O R' + ester H2O water (R represents a hydrocarbon group) You will synthesize the ester found in banana oil; the ester's common name is isoamyl acetate, and its boiling point is 142ºC. The purity of your synthesized ester will be checked by the boiling point. Part I. Preparation of Ester (1 week prior to lab) 1. Esters are made from the reaction of carboxylic acids with alcohols. Measure about 15 ml of acetic acid (ethanoic acid), and transfer to a dry 125 ml Erlenmeyer flask. 2. Slowly add about 20 drops of concentrated H2SO4 and mix thoroughly. 2 3. Add about 13 ml isoamyl alcohol (3-methyl-1-butanol), stopper, and mix thoroughly. (Avoid wetting the stopper – concentrated H2SO4 reacts with it.) Label the flask with your name(s) and store in your lab drawer for the next lab session. Part II. Separation of Ester (Second Week) 1. Pour the solution you prepared into 50 to 75 ml of ice cold H2O in a 250 ml beaker and mix thoroughly. The beaker contains two layers: (1) the bottom layer contains water and substances soluble in H2O and (2) the upper layer contains organic compounds insoluble in H2O (your ester and probably some unreacted acid or alcohol (if they are not very water soluble). To separate the lower aqueous layer from the organic layer containing your ester, you will use a separatory funnel. (See Fig. 1 below.) Figure 1: Separatory funnel a. Transfer your mixture (in portions if necessary) to the separatory funnel. Stopper. (Be sure the stopper fits snugly – check to see if it leaks.) Shake vigorously for a minute to insure thorough mixing of the layers, holding the stopper in, and periodically inverting the funnel (stopcock up) and cautiously opening the stopcock to release pressure. b. Support the funnel on a ring and allow the layers to separate and clarify. 3 c. Remove the stopper and open the stopcock to draw off and collect the bottom H2O layer. Pour it into the sink. Add 25 more ml of cold H2O to the funnel, mix again, let separate, and discard the bottom H2O layer. d. Add 25 ml of 5% Na2CO3 solution and leave the stopper off as gas is released. Swirl gently. After a minute, replace the stopper and invert, releasing pressure by opening the stopcock. (Be sure it's pointed away from other students!) Mix thoroughly, being sure to release the pressure frequently. It will react with any residual acid to give CO2 gas. Draw off and pour the bottom layer into the sink. e. Transfer the ester (top layer) through the mouth of the separatory funnel to a dry Erlenmeyer flask containing about 0.5 g anhydrous sodium carbonate and about 0.5 g anhydrous magnesium sulfate (both absorb water). f. Heat the mixture gently on a hot plate (don't let it boil) for 10 minutes, occasionally swirling well to allow the sodium carbonate to neutralize traces of H2SO4 and to allow both dehydrating agents to absorb traces of water. Carbon dioxide evolution indicates that acid is being neutralized. While you are waiting, proceed with Part III, No. 1, below. Part III. 1. Distillation of Ester Set up the distillation apparatus shown on the below (Fig. 2). The inside of all the glassware must be clean and dry. If it is necessary to wash any, use acetone as a final rinse since it evaporates much more quickly than H2O. Figure 2: Distillation apparatus VWR Digital Thermometer 4 Caution: the pieces in the kit do not necessarily remain stuck together! (Clamp them!) The thermometer must go up to at least 150ºC. Use a gentle rotary motion and insert it in the grey rubber piece. The distilling pot should be no bigger than 100 ml. Use a wire screen on a ring under the pot. Use an Erlenmeyer flask at first to collect the forerun, then a graduated cylinder to collect purified ester. A hose should connect the lower condenser outlet to a faucet, and another hose should drain the upper outlet into the trough in the counter top. 2. After the mixture has heated for 10 minutes, carefully pour the ester away from the solids through a funnel into the 50 or 100 ml distilling pot and add two boiling chips to make it boil more smoothly. 3. Adjust the thermometer so the bulb (or tip of the probe if digital) is just below the sidearm of the distillation flask. 4. Have the instructor check your setup. 5. Read this entire part before proceeding. Gently turn on the condenser water. Heat the distilling flask (pot) with a small flame. When the liquid begins to boil, adjust the flame so the liquid drips into the receiving Erlenmeyer flask at about 1 to 2 drops per second (no faster!). (Remove the burner, if necessary, or increase the flame to maintain the desired rate.) The point of distilling is to separate your ester from impurities (which boil at other temperatures) The boiling point of your ester is 142ºC, while your acetic acid has a boiling point of 118ºC, and the alcohol has a boiling point of 128.5ºC. You will save the liquid that distills within 4ºC of your ester's boiling point, and discard the lower boiling liquid you are collecting now. Therefore, stop heating when the temperature is about 138ºC and replace the receiving Erlenmeyer flask with a dry 25 ml graduated cylinder. Discard the liquid in the Erlenmeyer flask. 6. 7. Continue distilling and collecting the ester until you only have a milliliter or so of liquid left in the distilling flask (don't distill dry!) or until the temperature reaches more than 146ºC. Turn off the flame and water. Record the ester's volume. 5 Name Date Report for Experiment: Banana Oil Boiling range over which ester was collected: Volume of the ester collected: Questions and calculations: 1. 2. a. Write an equation for the preparation of your ester from an alcohol and an acid using structural formulas. b. Give the IUPAC name of your ester. c. Considering the structural formula of your ester, would you expect it to be soluble or insoluble in H2O? Explain. Look carefully at the condenser, (see p. 3). Explain what it does and how it works. 6 3. The results of organic synthesis experiments are often reported in terms of percent yield. Percent yield = actual amount of product theoretical yield 100 (where theoretical yield = amount of product you would have obtained if the reaction had proceeded to completion (all reactants converted to products). Since organic reactions rarely proceed to completion – they are equilibrium reactions – do not expect to get 100% yields! Calculate your percent yield (amounts of your reactants were chosen so that your theoretical yield of ester was 40 ml).