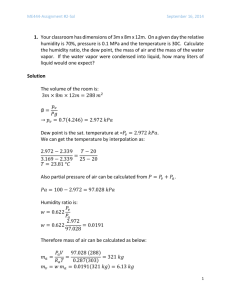
Biomass Combustion Study Pack for WEBCT Contract
advertisement

University of Portsmouth Biomass Combustion Study Pack for WEBCT Contract by Prof. M. R. I. Purvis S. O. Santos © February 2002 Department of Mechanical and Manufacturing Engineering University of Portsmouth United Kingdom of Great Britain Table of Content Learning Objectives 1 Study Topic 1 1.1. What is Biomass? 1 1.2. Importance of biomass as energy source 1 1.3. How do we convert biomass to energy 4 1.4. Biomass Composition and Energy Content 5 1.5. Advantages and Disadvantages of Using Biomass 8 1.6. Principles of Biomass Combustion 1.6.1. Devolatilisation 1.6.2. Char Combustion 8 10 11 1.7. 17 17 18 20 Combustion Equipment 1.7.1. Fixed Bed Combustion 1.7.2. Fluidised Bed Combustion 1.7.3. Suspension Burning 1.8. Thermal Efficiency 21 1.9. Combustion Air 22 1.10. Products of Combustion 24 1.11. Environmental Issues and Biomass Combustion 1.11.1. Effects of Pollutants 1.11.2. Quantification of Emissions 1.11.3. Nitrogen Oxides 25 26 27 27 1 Learning Objectives On the completion of this study pack, you should be able to: describe properties of biomass materials and their importance to the design of combustion plant understand principles of biomass combustion explain the importance of biomass as a sustainable source of energy recognise the environmental impact of biomass combustion identify significant literature to aid biomass combustion modelling Study Topic 1.1. What is Biomass? Biomass is… ¾ plant and other growing species capable of being used as fuel. ¾ organic material mainly composed of carbohydrate and lignin compounds, the building blocks of which are the elements carbon, hydrogen and oxygen. ¾ stored form of solar energy relying on the process of photosynthesis. Some examples of biomass are… fuel wood sugar cane bagasse switch grass rice hull coconut shells 1.2. tree barks wheat straw corn cobs vineyard pruning almond shell Importance of biomass as energy source As late as the mid 1800s, biomass supplied the vast majority of the world’s energy and fuel needs (as shown in Figure 1). It only started to phase out in industrialised countries as the fossil fuel era began, slowly at first and then at a rapid rate (Klass, 1998). But with the onset of the first major oil crisis in the early 1970s and the increasing stringency of regulations on pollution emissions, biomass was again realised by many governments and policy makers to be a viable, domestic energy resource which has the potential for reducing oil consumption and imports and limiting various air pollutants such as CO2, SOx, NOx and many trace emission. A case in point is shown in Figure 2 which indicates the Page 1 Figures 3 and 4 describe world’s energy consumption. Figure 3 shows the share of the different fuels to the world’s primary energy supply. It is seen that the global utilisation of combustible renewables and wastes, which includes biomass as a primary source of energy has increased from 677 Mtoe to 1,063 Mtoe during the period of 1973 to 1998. Energy Inputs (%) energy distribution for Sweden for the year 1973 and 1998. The figure illustrates the contribution of combustible renewables and wastes (which also includes biomass fuel) to the primary energy supply in Sweden, i.e. increases from 8.9% to 14.8% between 1973 and 1998. Likewise, it also shows the contribution of oil to the primary energy supply, i.e. decreasing from 44.9% in 1973 to 35.7% in 1998. Calendar Year Figure 1: Evolution of the primary energy structure. This figure shows the contribution of various types of fuel to the global energy input from 1860 to 1990 (Dupont-Roc et al, 1996) Sweden’s Total Primary Energy Supply 1998 1973 Com bustible Renew ables & Waste 8.9% Hydro 13.0% Coal 4.1% Others** 0.7% Coal 5.0% Oil 30.3% Gas 1.3% Hydro 12.0% Nuclear 1.5% Oil 72.4% Com bustible Renew ables & Waste 14.8% Nuclear 35.9% 39.3 Mtoe ** Others include geothermal, wind, solar, heat etc… 52.5 Mtoe Figure 2: The share of various fuels to the total primary energy supply of Sweden between 1973 and 1998. The total energy used is 39.3 and 52.5 million tonne of oil equivalent (Mtoe) for 1973 and 1998 respectively. (IEA, 2000a) World’s Total Primary Energy Supply 1973 6,043 Mtoe 1998 ** Others include geothermal, wind, solar, heat etc… 9,491 Mtoe Figure 3: The share of various fuels to the world’s total primary energy supply between 1973 and 1998. The total energy used is 6,043 and 9,491 million tonne of oil equivalent (Mtoe) for 1973 and 1998 respectively. (IEA, 2000b) Page 2 Gross National Product per capita (US$ / capita-year) Figure 4 presents the relationship between gross national product (GNP) per capita to energy consumption per capita. The figure shows a clear difference in the energy consumption of developed and developing countries. For many developing countries, fuel wood is the primary source of energy particularly in the domestic sector. Figure 5 shows the contribution of wood energy in various Asian countries. Energy consumption per capita (kg of oil equivalent / capita-year) Figure 4: Gross national product vs energy consumption of selected countries (Klass, 1998) Share of wood energy in the total energy consumption in various Asian countries Figure 5: Share of wood energy in total energy consumption in various Asian countries (FAO United Nation, 1997) Page 3 Question 1: 1.1. From Figure 2, calculate the energy consumption per capita of Sweden in 1998 assuming that the population is about 8.8 million. 1.2. From Figure 3, draw the histogram showing the changes in World’s energy supply between 1998 and 1973 in terms of the following categories: fossil fuel, non-fossil fuel and biomass fuel (Note that nonfossil fuel includes nuclear, wind, solar, geothermal and hydro). 1.3. How do we convert biomass to energy Biomass materials are processed in various ways to produce heat, chemicals and other types of fuels. The different conversion processes involved are generally classified as either: (i.) thermal conversion process or (ii.) biochemical conversion process. Figure 6 shows a schematic diagram summarising how biomass is converted into energy and heat. Direct Combustion Thermal Conversion Gasification Liquefaction Pyrolysis Various Gaseous and Liquid Fuels, Tars and Charcoal Biomass Energy and Heat Anaerobic Processes Methane Fermentation Ethanol Biological Conversion Figure 6: Schematic diagram showing how biomass are converted to produce heat and energy. Thermal conversion processes include combustion, liquefaction, gasification and pyrolysis, while biochemical conversion processes include anaerobic digestion and fermentation. In this study pack, the direct combustion of biomass is emphasised. Table 1 presents some of the common products produced during gasification, liquefaction or pyrolysis of biomass. Table 2 presents the product yield from the thermal decomposition of Birch, Pine and Spruce woods heated over an 8 hour period to a final temperature of 400oC. Table 1: Some of the common products produced during gasification, liquefaction and pyrolysis of biomass Solid Products charcoal, ash Liquid Products water, tar, volatile acids, alcohols, aldehydes, esters, ketones Gaseous Products H2, CO, CO2, CH4, C2H4, C2H6 Page 4 Table 2: Product yields from thermal decomposition of Birch, Pine and Spruce woods heated over an 8 hour period to a final temperature of 400oC. (Klass, 1998)a Products Birch (wt%) Pine (wt%) Spruce (wt%) Gases H2 CO CO2 CH4 C2H4 0.03 4.12 11.19 1.51 0.21 0.03 4.10 11.17 1.49 0.14 .03 4.07 10.95 1.59 0.15 Charcoal 33.66 36.40 3.43 21.42 3.75 10.42 22.61 10.81 5.90 23.44 10.19 5.13 7.66 1.83 0.50 1.63 1.13 3.70 0.89 0.19 1.22 0.26 3.95 0.88 0.22 1.30 0.29 0.94 1.09 0.38 Liquid products water settled tar soluble tar volatile acids alcohols aldehydes esters ketones Losses a Data are obtained by Klass (1998) from work of Nikitin et al (1962) & Bagrova and Kozlov (1958). Volatile acids are calculated as acetic acids; Alcohols as methanol; Aldehydes as formaldehydes; Esters as methyl acetate and Ketones as acetone. 1.4. Biomass Composition and Energy Content In a similar way to coal, biomass materials are also characterised according to their Ultimate Analysis, Proximate Analysis and their Gross Calorific Values. Table 3 presents the Ultimate Analysis of some biomass materials and UK coal; Table 4 presents their Gross Calorific Values and Proximate Analysis. Unlike coal, wood and other biomass contains complex carbohydrates and lignin. Table 5 presents the chemical composition of softwood and hardwood. Figures 7A to 7C illustrate the chemical structure of lignin, cellulose and coal respectively. These figures show that coal consists mostly of complex aromatic compounds while most biomass, which consists of carbohydrates and lignin (the only source of aromatics), would have less complex aromatic compounds and more oxygen and hydrogen atoms attached. This explains why biomass has higher atomic H/C and O/C ratio than coal. Table 3: Ultimate Analysis of Some Biomass Fuels and UK Coal (CRE, 1995; Rossi, 1984) Fuel Rice hulls Gin Trash Peach pits Black Oak Red Alder Red Alder Bark Douglas-fir Douglas-fir Bark W. Hemlock Markham Main Daw Mill Cynheidre Taff Merthyr Fuel Types Agricultural Agricultural Agricultural Hardwood Hardwood Hardwood Softwood Softwood Softwood UK Coal UK Coal UK Coal UK Coal Weight Percent by Element (Dry Basis) Atomic Ratio C H O N S Ash H/C O/C 38.0 42.8 49.1 49.0 49.6 50.9 50.6 54.1 50.4 78.7 76.8 93.2 88.4 4.4 5.1 6.4 6.0 6.1 5.5 6.2 6.1 5.8 5.0 4.5 2.8 4.0 35.5 35.4 43.5 43.5 43.8 40.7 43.0 38.8 41.4 8.9 10.9 0.3 1.2 0.8 1.5 0.5 0.2 0.1 0.4 0.1 0.2 0.1 1.7 1.2 1.0 1.4 0.1 0.6 0.0 0.0 0.1 -0.0 tr. 0.1 1.5 1.4 0.6 0.7 21.00 14.69 0.50 1.34 0.41 2.50 0.10 1.00 2.20 3.71 4.69 1.83 4.03 1.36 1.43 1.55 1.47 1.48 1.30 1.47 1.35 1.38 0.76 0.71 0.37 0.55 0.69 0.62 0.66 0.67 0.66 0.60 0.64 0.54 0.62 0.08 0.11 0.00 0.01 Page 5 Table 4: Proximate Analysis of Some Biomass Fuels and UK Coal (Rossi, 1984; CRE, 1995) Fuel Rice hulls Gin Trash Peach pits Black Oak Red Alder Red Alder Bark Douglas Fir Douglas Fir Bark W. Hemlock Markham Main Daw Mill Cynheidre Taff Merthyr Fuel Types Agricultural Agricultural Agricultural Hardwood Hardwood Hardwood Softwood Softwood Softwood UK Coal UK Coal UK Coal UK Coal Gross Calorific Value (MJ/kg) 14.89 15.58 19.42 18.65 19.30 19.44 20.37 21.93 19.89 33.60 32.82 35.64 36.50 Proximate Analysis (weight per cent, oven dry basis) Volatile Matter (VM) Fixed Carbon (FC) Ash VM/FC Ratio 63.60 75.40 79.10 85.60 87.10 87.00 87.30 73.60 87.00 36.14 38.02 4.67 12.80 15.80 15.40 19.80 13.00 12.50 19.70 12.60 25.90 12.70 60.15 57.29 93.50 83.17 20.60 9.20 1.10 1.40 0.40 3.00 0.10 0.50 0.30 3.71 4.69 1.83 4.03 4.00 4.90 4.00 6.60 7.00 3.90 6.90 2.80 6.80 0.60 0.66 0.05 0.15 Figure 7-A: Hypothetical representative structure of a coniferous lignin (Antal, 1982) Figure 7-B: Hypothetical representation of cellulose structure (Antal, 1982) Figure 7-C: Hypothetical representation of coal structure (Wender et al, 1981) Page 6 Table 5: Chemical Composition of Wood (%)a (Tsoumis, 1991) Components Softwood Hardwood Holocellulose 59.8 - 80.9 71.0 - 89.1 Cellulose 30.1 - 60.7 31.1 - 64.4 12.5 - 29.1 18.0 - 41.2 4.5 - 17.5 12.6 - 32.3 Lignin 21.7 - 37.0 14.0 - 34.6 Extractives (hot water) 0.2 - 14.4 0.3 - 11.0 Extractives (cold water) 0.5 - 10.6 0.2 - 8.9 Extractives (ether) 0.2 - 8.5 0.1 - 7.7 Ash 0.02 - 1.1 0.1 - 5.4 Polyoses b Pentosans b a. Ranges of values are derived from 153 Temperate Zone species. As a rule, extractives are determined from oven dry basis and others are determined from oven dry and extractives free basis. b. Hemicellulose Another important property of biomass is the moisture content. Siau (1984) has noted that water in wood can be contained in the pores or chemically bound within the structure of the biomass. The moisture present in most biomass materials can be classified as: o bound water o free water o water vapour Table 6 summarises the total moisture content of some biomass fuels. Table 6: Moisture Contents of Some Biomass Fuels (Rossi, 1984) Fuel Range of Moisture Content (%) Woody Biomass Bark Chips Cull material Hog fuel Planer shavings Sander dust Sawdust 30 - 60 40 - 50 40 - 70 30 - 60 8 - 19 2-6 40 - 55 Agricultural Wastes Gin trash Grape pomace Nuts Orchard pruning Peach pits Tomato pomace Rice hulls Vineyard pruning 7 - 12 50 - 60 10 - 35 20 - 40 30 - 40 50 - 75 7 - 10 20 - 40 Question 2: 2.1. Comment on whether ash or moisture would help or hinder the combustion of wood in a furnace. 2.2. From Table 3 and 4, tabulate the following for coal (not including lignite), agricultural biomass, wood, and tree barks: a.) volatile to fixed carbon ratio b.) atomic hydrogen to carbon ratio c.) atomic oxygen to carbon ratio d.) gross calorific values Page 7 1.5. Advantages and Disadvantages of Using Biomass Advantages ¾ lower sulphur and nitrogen contents as compared to coal and fuel oil results in lower SOx and NOx emissions. ¾ sustainable management of biomass will results in a reduction of CO2 emission by displacement of fossil fuels (CO2 produced during combustion are considered CO2 neutral.) ¾ provide savings for most developing countries by displacing imported fossil fuels Disadvantages ¾ have low thermal intensity as compared to fossil fuels; therefore, for a specified plant duty, biomass mass flows are greater than those for coal. ¾ most biomass has low density and bulk density and therefore requires larger equipment for handling, storage and burning. ¾ biomass materials are normally high moisture content therefore reducing combustion efficiency. Question 3: 3.1. Identify the main pollutants from biomass combustion and their related impact? 3.2. Describe the regulation for the control of smoke emission from stationary combustion plant in Sweden? 1.6. Principles of Biomass Combustion Burning of biomass to obtain heat and light is one of the oldest biomass conversion processes known to mankind. Complete combustion (i.e. incineration, direct firing) of biomass consists of (a.) rapid chemical reactions (oxidation) of biomass and oxygen, (b.) the release of energy and (c.) the simultaneous formation of the ultimate oxidation products of organic matter (i.e. CO2 and water). The basic stoichiometric equation for the combustion of wood, represented by the empirical formula of cellulose, [C6H10O5]n, is given by: [C6H10O5]n + 6nO2 → 6nCO2 + 5nH2O The combustion of lump biomass can be simply described by the burning of a single biowet core pyrolysis front at r = rp dry shell volatiles from the particle water from the particle drying front at r = rd char layer flame region Figure 8: Schematic diagram showing the combustion of a small biomass particle (i.e. with a 20 mm diameter) Page 8 mass particle as shown in Figure 8. ¾ Biomass fuel follows a similar sequence of processes as applies to the combustion of coal. These include the following: o Drying o Devolatilisation o Char combustion o Volatile combustion ¾ Assuming that a 20 millimetre sized particle is burned in a furnace, the combustion mechanisms of the biomass particle can be classified into two phases namely: preignition phase and post-ignition phase. For the combustion of this biomass particle, the following stages may occur simultaneously or concurrently. The basic steps are described as follows: o Step 1: Drying of the biomass particle immediately commences as the particle is introduced into the hot combustion environment. Due to the higher permeability of the vapour phase, a wet-dry interface (as indicated by the drying front at r = rd as shown in Figure 7) is formed within the particle. o Step 2: As the dry shell starts heating up, devolatilisation commences with the breaking up of the solids structure. o Step 3: As the volatile release becomes more rapid, ignition of the volatiles are assumed to occur in the gas phase. Following ignition, volatiles burn in a thin flame enveloping the particle surface. It is believed that this phenomenon prevents oxygen reaching the charred surface and the flame energy promotes the drying and devolatilisation of the particle. o Step 4: As all the moisture is vaporised, volatilisation may still continue. Several workers (Veras et al, 1999; Saastamoinen et al, 1993; Simmons, 1983) have indicated that there is a possible simultaneous occurrence of devolatilisation and char combustion at this stage of the process. o Step 5: As the volatiles become depleted, the volatile flame collapses and oxygen is permitted to attack the charred surface therefore leading to the ignition and burnout of the residual char. ¾ Among the complex processes involved during combustion, the devolatilisation of the biomass materials and the subsequent combustion of the char produced during devolatilisation are seen to be the most important process that define parameter for the design of a furnace. This will be described in more detail in the succeeding sections. Question 4: 4.1. How does the reactivity of the fuel particle vary with biomass properties? 4.2. What additional physical processes are involved if the particle is burned in a fuel bed? Page 9 1.6.1. Devolatilisation The devolatilisation of biomass materials involves several complex processes of thermal decomposition and can be generalised as: ¾ Removal of bound moisture and some volatiles ¾ Breakdown of hemicellulose; emission of CO and CO2 ¾ Exothermic reaction causing the wood temperature to rise from 250 to 360oC; emission of methane and ethane. ¾ External energy is required to continue the process (i.e. breakdown of cellulose and lignin). The products of biomass devolatilisation are generally classified into gas, tar and char (Di Blasi, 1993). The distribution of these products depends on various parameters such as biomass composition, reaction temperature, heating rate, particle size and catalyst used. Usually, under a fast devolatilisation condition yields more gases than solids. The rate of mass loss during devolatilisation can be determined using various models. The simplest among these models assumes an Arrhenius form of equation with a first order reaction represented as: − dm v = Am v exp( − E / RT ) dt (Eqn. 1) where mv A E R T = mass of volatiles remaining (kg) = Arrhenius constant = activation energy (kJ/kmol) = universal gas constant (kJ/kmol-K) = temperature (K) The activation energy (E) and pre-exponential constant (A) vary considerably depending upon the pyrolysis conditions and fuel type as shown in Table 7. Some other models assume two competing reactions or a series of competing and concurrent steps. Figure 9 presents an outline of a commonly used reaction scheme describing the devolatilisation of biomass. Table 7: Kinetic data for devolatilisation assuming first order reaction kinetics (Di Blasi, 1993) Temperature (K) E (kJ/mol) A(s-1) Reference α - cellulose Sample 550 - 1000 79.4 1.7 x 104 Kanury (1972) Cellulose 600 - 850 100.5 1.2 x 106 Tabatabaie et al (1989) -3 Beech sawdust 450 - 700 18 (T < 600) 5.3 x 10 Barooah and Long (1976) Beech sawdust 450 - 700 84 (T > 600) 2.3 x 104 Barooah and Long (1976) Cellulose Lignin 520 – 1270 520 - 1270 166.4 141.3 3.9 x 10 11 Lewellen et al (1978) 8 Min (1977) 1.2 x 10 9 Hemicellulose 520 - 1270 123.7 1.45 x 10 Wood 321 - 720 125.4 1.0 x 108 Nolan et al (1973) Min (1977) Almond Shell 730 - 880 95 - 121 1.8 x 106 Font et al (1990) Page 10 i.) Simple Single Step Reaction k Biomass → Volatiles + Char Biomass k → a Gases + b Tars + c Char ii.) Multiple Single Step Reactions k1 Product (1) k2 Product (2) k3 Biomass Product (3) • • • • • • • • • • • • Product (i) iii.) Multi Step Reaction k1 Biomass k2 Gases + Volatiles Flaming Combustion k5 Tar k4 k3 Char + Gases Glowing Combustion Figure 9: A common reaction scheme used in biomass pyrolysis (Adapted from Di Blasi, 1993) Recent development in the modelling of biomass devolatilisation involves the use of network models such as the bio-FG DVC [Functional Group – Depolymerisation, Vaporisation, and Crosslinking] (Chen et al, 1998) and bio-FLASHCHAIN (Niksa, 2000). These network models are developed based on an assumed structure of the biomass and a set of kinetic data from a fuel library. These data are determined from various experiments and modelled on the principle of distributed activation energy. For example, bio-FG DVC describes the devolatilisation process in two steps. The first step involves the evolution of gases and tars based on the breakaway of various functional groups from their parent structure. The second step describes the reactions of the solid materials based on a Monte Carlo simulation. The model was applied to several types of biomass such as wheat, corn stalk and wood. Further details of this model are described in the literature (Solomon et al, 1992; Chen et al, 1998). Illustrative Example (#1): A small wood particle has a temperature of 800 K. Find the time required to devolatilise 90% of the volatile mass, assuming that it follows a first order reaction and with Arrhenius constant (A) = 7 x 107 and activation energy (E) = 125 kJ/mole. Solution to Illustrative Example (#1): Using the equation for a first order reaction dm v − = Am v exp( − E / RT ) dt Rearranging m t dm v ∫m − m Aexp(-E / RT) = ∫t dt v v2 v1 2 1 Integrating m − ln v2 m v1 = t − t 2 1 Aexp(- E/RT) since mv2 = 0.1 mv total & mv1 = mv total Substituting 0.1 m v total − ln m v total = t2 − 0 7 x10 7 exp(-125,000/8.314/800) thus time (t2) required is 4.78 sec. Page 11 1.6.2. Char Combustion The final step in solid fuel burning is the char combustion. When devolatilisation is complete, char and ash remain. Oxygen or other oxidisers (i.e. CO2, H2O) can diffuse through the external boundary layer into the char particle and react with the char surface, (Generally, char is greater than 95% carbon). Basically, the char combustion process involves the following gas-solid reaction processes (Laurendeau, 1978): o Transport of the oxygen molecules or oxidisers to the surface by convection and/or diffusion. o Adsorption of the oxidiser molecule on the surface. o Reaction of the adsorbed oxidiser molecule with the surface, reaction of the surface itself or reaction of the surface and gas phase molecules. o Desorption of the combustion product from the surface. The burning rate of char mainly depends on both chemical rate of the carbon-oxygen (oxidiser) reaction at the surface and the rate of diffusion of the oxygen (oxidiser) into the boundary layer and into the internal voids within the char. Likewise, burning rate also depends on the oxygen (oxidiser) concentration, gas temperature, Reynolds Number, and char size and porosity. Biomass char is known to be highly porous [i.e. wood char is about 94% porous] (Williams et al, 2000). This therefore results in an internal surface area of around 10,000 m2/g (for wood char with 90% porosity). This property of biomass char strongly affects the combustion rate of the char. For engineering purposes, the global reaction rate of order ‘n’ with respect to oxygen is used to calculate the char burning rate and is given by: M dm c = −i c MO dt 2 A p k c ρ O (s ) n ( 2 ) (Eqn. 2) where i , MC, MO2, Ap, kc, ρ O (s ) and n are the stoichiometric ratio (moles of carbon per mole of oxygen; i = 2 if product is CO and 1 if product is CO2), molecular mass of carbon, molecular mass of oxygen, total surface area, kinetic rate constant, concentration of oxygen at the char surface and reaction order respectively. 2 Assuming that reaction order (n) is equal to one, thus: [ ] A p k c ρ O (s ) = A p hD ρ O (∞ ) − ρ O (s ) 2 2 2 (Eqn. 3) where hD and ρ O (s ) are the mass transfer coefficient and ambient oxygen concentration respectively. Therefore, burning rate can be simplified to: 2 M dm c = −i c MO dt 2 A p k e ρ O (s ) 2 (Eqn. 4) where ke is the effective kinetic rate constant that can be expressed as: k e = hD k c hD + k c Page 12 To aid the understanding of the char combustion process, burning of non-porous pure carbon is used as an example. There are two limiting conditions available in describing the combustion of a spherical carbon particle. These conditions are defined in: o the one-film model and o the two-film model Figures 10 and 11 present the species and temperature profiles of a burning carbon particle assumed to follow one-film and two-film models respectively. Yi, T Gas-Solid Interface (Carbon Surface) Yi – concentration of species i. T – temperature r - radius YCO2 (s) YO2 (∞) YCO2 (r) Tp YO2 (r) Ts T(r) T(∞) YO 2 (s) r rs 0 Figure 10: Species and temperature profiles for one film model of carbon combustion assuming that CO2 is the only product of combustion at the carbon surface (Turns, 1998). Yi, T Carbon Surface Yi – concentration of species i. T – temperature r - radius Flame Sheet YCO (s) YO 2 (∞) T(r) Tp Ts YCO (r) T(∞) YCO2 (r) YO2 (r) YCO2 (s) 0 rs r Figure 11: Species and temperature profiles for two-film model of a burning spherical carbon particle (Turns, 1998) Page 13 At the surface, carbon can be attacked by O2, CO2 or H2O as shown in the following reactions: C + O2 ⇒ CO2 C + ½O2 ⇒ CO C + H2O ⇒ CO + H2 C + CO2 ⇒ 2CO Then CO diffuses away from the surface through the boundary layer where it can react with the inward diffusing O2 as: CO + O2 ⇒ CO2 For the one-film model (as shown in Figure 10), it can be seen that the CO2 concentration is a maximum at the carbon surface and is approximately zero far from the particle surface. Conversely, O2 concentration is at minimum at the surface. If the chemical reaction rate is much greater than the oxygen diffusion rate, then the O2 concentration approaches zero; otherwise, if the diffusion rate is much faster than the chemical reaction rate, then O2 concentration at the surface will be appreciable. Since, for this model, it is assumed that there is no reaction involved in the gas phase, the temperature is at a maximum on the carbon surface and then falls monotonically to T∞. On the other hand, for the two-film model (as shown in Figure 11), it can seen that a flame sheet is present at some distance away from the surface; and the CO2 species attacking the carbon surface is reduced to yield CO. The maximum temperature is located around the interface of the flame sheet. Likewise, O2 and CO are a minimum at the interface of the flame sheet and CO2 is a maximum. In the one-film model the following assumption are usually made (Turns, 1998): o Burning process is quasi-steady state. o Spherical carbon particle burns in a quiescent, infinite ambient medium that contains only oxygen and an inert gas such as nitrogen. There are no interactions with other particles and effects of convection are ignored. o At the particle surface, the carbon particle reacts kinetically with oxygen to produce only CO2. o The gas phase only consists of O2, CO2 and inert gas. The O2 diffuses inward, reacts with the surface to form CO2 which then diffuses outward. The inert gas forms a stagnant layer as in the Stefan problem. o Gas phase thermal properties (i.e. conductivities, specific heat, product of the density and mass diffusivity) are constant. Lewis number is equal to one (Le = k/ρcpD = 1). o Particle temperature is uniform and radiates as a gray body to the surroundings without participation of the intervening medium. Using a mass balance and assuming that diffusion of O2 and CO2 into and out of the carbon particle follows Fick’s Law, the burning rate of carbon can be calculated as (Turns, 1998): Page 14 &c = m (Y O2 ∞ −0 ) (Eqn. 5) R kin + R diff where R kin ≡ 1 k kin ν s( 1− film ) RTs = 4πrs2 MWmix k c P (Eqn. 6) where ν s( 1− film ) , R, Ts, rs, MWmix, kc and p are the stoichiometric ratio of the reacting species at the surface for the one-film model (32/12), universal gas constant (8.314 J/mol-K), carbon surface temperature (K), radius of the particle, molecular mass of gas mixture (kg/kg-mole) and pressure (Pa) respectively. and R diff ≡ (ν s ( 1− film ) + YO s 2 ) 4πrs ρD (Eqn. 7) where YO s , ρ and D are oxygen concentration at the surface, gas mixture density and diffusivity respectively. 2 Likewise, at the carbon surface, the burning rate is calculated as (Turns, 1998): & c = k kin YO 2s m (Eqn. 8) For the two-film model, the following assumptions are used: o Burning process is quasi-steady state o Spherical carbon particle burns in a quiescent, infinite ambient medium that contains only oxygen and an inert gas such as nitrogen. There are no interactions with other particles and effects of convection are ignored. o At the particle surface, the carbon particle reacts kinetically with CO2 to produce only CO. o The gas phase only consists of O2, CO2 and inert gas. The O2 diffuses inward, reacts with CO along the first gas film (gas layer before the flame sheet interface) to form CO2 which then diffuses outward. The inert gas forms a stagnant layer as in the Stefan problem. o Gas phase thermal properties (i.e. conductivities, specific heat, product of the density and mass diffusivity) are constant. Lewis number is equal to one (Le = k/ρcpD = 1). o Particle temperature is uniform and radiates as gray body to the surroundings without participation of the intervening medium. In this case, the burning rate of carbon surface is calculated as (Turns, 1998): a.) At the carbon surface & c = k kin YCO s m 2 (Eqn. 9) where k kin(2-film) = 4πrs2 MWmix k c P ν s( 2− film ) RTs (Eqn. 10) Page 15 b.) At the gas phase, the burning rate is calculated as & c = 4πrs ρD ln(1 + B ) m and ( (Eqn. 11) ) −1 ν 2YO ∞ − s( 2− film ) YCO s ν s ( 2 − film ) B= −1 ν ν s( 2 − film ) − 1 + s( 2 − film ) YCO s ν s ( 2 − film ) 2 2 ( (Eqn. 12) ) 2 where ν s( 2− film ) , YO ∞ and YCO s are the stoichiometric ratio at the surface in the two-film model (44/12), oxygen concentration at the free stream and carbon dioxide concentration at the surface. 2 2 Illustrative Example (#2): Using the one-film model, estimate the burning rate of a 90 µm carbon particle burning in a still air ( YO ∞ = 0.233) and 1 atm. 2 The particle temperature is 1750K and the kinetic rate constant is assumed to be 13.9 m/s. Assume that the mean molecular mass of the gas mixture surrounding the particle is 30 kg/kmol and the mass diffusivity is estimated using the value for the mass diffusivity of CO2 in N2 which is 1.6 x 10-5 m2/s at 120oC. Also what is the prevailing combustion regime? Solution to Illustrative Example (#2) Calculation Procedure: 1.5 Note: calculation for the onefilm or two-film model usually involves iteration. To solve for the burning rate, the following procedure is used: a.) Calculate Rdiff and initially assuming that YO s = 0 2 b.) Calculate Rkin and then solve for the burning rate. c.) Using the equation for burning rate at the surface, calculate the value of YO s . 1750 K D= 1.6 x 10−5 m 2 /s = 1.19 x 10-4 m 2 /s 393 K Assuming Solution: ρ = = P MWmix RTs (101,325Pa)(30 kg/kmol) (8314 J/kmol - K)(1750 K) = 0.209 kg/m3 for the time being. (ν s( 1− film ) + YO s ) = R diff 2 4πrsρD 4π( 45 x 10-6 R kin = ν s( 1− film )RTs 4πrs2 MWmix k c P (3212)(8314)(1750) = 4π( 45 x 10-6 )2 (30) (13.9)(101,325) = 3.61 x 107 s/kg = Recalculating Rdiff R diff - 2nd iter R diff -1st iter. ( ( (YO ∞ − 0) 2 R kin + R diff (0.233 − 0) = 1.25 x 10-9 kg/s 3.61 x 107 + 1.50 x 108 Using the equation for burning rate on the surface, & c = k kin YO s m 2 ) ) ν s( 1− film ) + YO2 s 2nd iter. = ν s( 1− film ) + YO2 s 1st iter. = )(0.209)(1.19 x 10-4 ) = 1.50 x 108 s/kg &c = m & c = (3.61 x 107 )(1.25 x 10-9 ) = 0.0452 YO 2s = R kin m (3212 + 0) = 2 d.) Recalculate Rdiff.and compare the values of previous Rdiff YO 2 s ≈ 0 rearranging (3212 + 0.0452) (3212 + 0) 2nd iter. = 1.0169 1st iter. ∴ Rdiff (2nd iteration) = (1.50 x 108)(1.0169) = (1.52 x 108) Recalculate burning rate (2nd iteration) YO2 ∞ − 0 (0.233 − 0) &c = m = R kin + R diff 3.61 x 107 + 1.52 x 108 = 1.23 x 10-9 kg/s ( ) (diffusion controlled combustion regime) Since changes in the burning rate are less than 2%, no further iteration is required. Challenge Question Recalculate problem using the two-film model assuming that kc = 4.016 x 108 exp (-29,790/Ts) [m/s] and compare the result from one-film model. (Ans: 2.3784x 10-9 kg/s) Page 16 1.7. Combustion Equipment Biomass materials can be burned in many ways. They can be pulverised, crushed, chipped, cut, chopped and fed into one of the several types of furnaces or boilers available. Generally, biomass materials can be burned in a: Fixed bed Suspension fire Fluidised bed Fuel handling and feeding are some of the factors which need to be considered regarding how the biomass materials are burned. The nature of combustion system dictates the required fuel preparation. Fixed bed combustion systems require the least amount of fuel size reduction as compared to suspension burning or fluidised bed combustion. Typical biomass materials are difficult to crush or pulverise due to their fibrous nature thus most biomass materials are burned either in a fixed bed or a fluidised bed where uniform particle size is not a pressing requirement (Borman and Ragland, 1998). 1.7.1. Fixed Bed Combustion The traditional campfire is a classical example of the fixed bed combustion of biomass using the natural convection of air as the oxidiser. Normally this kind of combustion (simple pile burning method) is considered low intensity combustion (ie. low heat output per unit volume) and smoky. In order to increase the combustion intensity, improve control of heat output, and reduced emissions, force draft and grates are used. Fixed beds can be operated using co-current, cross-current or counter current air flow. This broad classification of fixed bed is based on how the fuel and air is introduced into the combustion system. Figure 12 shows how these three types of fixed bed combustion operate. It also illustrates the different reaction zones that can occur within the bed. Counter Current Co-Current Cross Current ash product gas product gas solid fuel product gas Ash Drying 1 Devolatilisation Gasification ash 3 Combustion 4 5 Combustion Devolatilisation Drying air Ash ash Gasification 2 solid fuel air 1: Drying 2: Devolatilization 3: Combustion 4: Gasification 5: Ash solid fuel air Figure 12: Schematic diagram of different fixed bed reactors Page 17 (a.) (b.) Figure 13: Some examples of fixed bed combustion. (a.) spreader stoker (b.) travelling grate stoker (Podolski et al, 1997) An example of counter current fixed bed combustion is the spreader stoker. This type of stoker operates in such a way that fuel is fed on top of the bed and air is introduced through several ports under the fuel bed. Figure 13(a) shows how the spreader stoker operates. For the counter current reactor, the fuel is generally fed on top of the bed and moves downward under the influence of gravity and moves counter to the flow of the gas stream. An example of a cross current combustion system is the travelling grate stoker as shown in Figure 13(b). In this type of stoker, fuel is fed through a hopper at one end of the grate while air is introduced through the small gaps that are situated all over of the grate. A typical example for co-current combustion is the underfeed stoker. In an underfeed stoker, both fuel and air are fed more or less in the same direction. This type of stoker is built with a single or multiple retort configuration. In a single retort, a ram or a screw pushes the fuel to the end of the stoker and upwards toward a tuyere block where air is introduced into the bed. On the other hand, a multiple retort stoker consists of a series of shallow, longitudinal retorts separated by rows of stepped air tuyeres. Figure 14 shows a schematic diagram of the underfeed stoker. 1.7.2. Heat Exchanger Tubes Combustion Gas to Chimney Figure 14: Schematic diagram of the underfeed stoker (Purvis et al, 2000) Fluidised Bed Combustion A fluidised bed is a bed of solid particles which are put into motion by blowing air upwards through the bed at a sufficient velocity to locally suspend the particles. The air blown through the bed has a velocity that is not great enough to blow the particle out of the bed and will make the bed appear like “boiling liquid”. The fluidised bed therefore exhibits buoyancy and a hydrostatic head. Figure 15 presents the schematic diagram of a fixed bed, a bubbling fluidised bed and a circulating fluidised bed. Page 18 Fixed Bed Bubbling Fluidised Bed Circulating Fluidised Bed Figure 15: Schematic diagram of a fixed bed, bubbling fluidised bed and circulating fluidised bed combustors (Borman and Ragland, 1998) Fluid bed combustion has been given much attention in recent times because of its advantages particularly in large scale systems. Combustion takes place in a cylindrical reactor in which air is dispersed through an orifice or a sintered plate at the bottom of the vessel. The air then passes through a bed of inert refractory pieces, particles of fuels, ash and other residual inorganic particles remaining from combustion therefore causing the bed to expand and to become “fluidised”. Smaller fuel particles burn rapidly on top of the bed while large fuel particles filter into the bed where they are dried and gasified. Most char is burned completely within the bed while the volatiles are burned partially in and partially above the bed. The fuel is fed either by a ram or by pneumatic means into the reactor clearance at around 900K. Fluidised bed combustion is suitable to burn high moisture content fuels because of its low heat input requirement and high thermal inertia. Materials such as limestone are often added to the bed to minimise pollutants in the flue gases. The constant motion of the bed allows good mixing between air and fuel, this improves combustion, reduces emissions and makes it possible to burn a wide range of fuels having different sizes, moisture contents and calorific values. Figure 16 presents a schematic diagram of a circulating fluidised bed combustion system. Figure 16: Schematic diagram of circulating fluidised bed combustion system (Borman and Ragland, 1998) Page 19 The air velocity in a bubbling fluidised bed is maintained slightly lower that the air velocity of a circulating fluidised bed to prevent particle carryover. Likewise, a bubbling fluidised bed requires a fuel feed point for approximately every 1 m2 of bed area for good mixing. Circulating fluidised bed combustors were developed to overcome particle carryover and facilitate fuel feeding. Fluidised bed can be operated either atmospheric or pressurised. Pressurised fluidised bed combustion systems are currently developed with an aim of directly powering a gas turbine using various solid fuels. 1.7.3. Suspension Burning Suspension furnaces burn pulverised fuel particles that are fed through a nozzles into the furnace volume which is large enough to allow burnout of the fuel chars. Figure 17 shows a schematic diagram of a pulverised fuel steam power plant. Solid Fuels Figure 17: Schematic diagram of a pulverised fuel combustion system (Borman and Ragland, 1998) As received, solid fuel is fed into a pulveriser (ie. ball mill) or a shredder. From the size reduction equipment, the pulverised fuel (coal or biomass) is piped with air to burners, where the fuel is mixed with pre-heated air from an air plenum (windbox). The volatile flames are stabilised by the burner while the chars are burned out in the radiant section of the furnace. The burner consists of a nozzle that delivers the fuel particle. The conveying air that delivers the pulverised fuel into the furnace is called the primary air and the air is about 20% of the required combustion air. The secondary or main air is supplied through a swirl vane surrounding the fuel nozzle. Figure 18 shows the schematic diagram of a fuel nozzle burner. The flame shape is controlled by the extent of secondary air swirl and the contour of the burner throat. The recirculation pattern which is set up inside and extends several throat diameters into the furnace provides a stabilisation zone for ignition and combustion of the volatiles. The velocity of the primary air and pulverised fuel should be greater than the speed of flame propagation so as to avoid flashback. The flame speed depends on the fuel-air ratio, the amount of volatile matter, ash in the fuel particle, particle size distribution, Page 20 Figure 18: Schematic diagram of a low NOx burner air pre-heat temperature and nozzle diameter. Fuel rich mixtures have the highest flame speeds. For low volatile and high ash fuel, the flame speed is low and the air from the secondary nozzle should be mixed appropriately to avoid flame instability. As mentioned earlier, wood and bark are more difficult to grind than coal because of their fibrous nature which is less friable than coal particles. However, pulverised biomass burners are noted to be economically attractive for retrofitting oil burners when an ample supply of pulverised biomass (i.e. sawdust) is available. Co-firing of biomass with coal in a pulverised combustor has been done to a limited extent (Sami et al, 2001). Several issues remain to be resolved when co-firing biomass and coal and some of these are: a.) Fouling and corrosion problems due to the alkalinity of biomass ash. b.) Burner stability and particle burnout should be considered due to differences in reactivity. c.) Fuel feeding mechanisms should be taken into account due to differences in physical properties. 1.8. Thermal Efficiency The thermal efficiency refers to the amount of heat recovered as useful heat divided by the heat input. This can be determined in two ways: a.) direct method b.) indirect method The Direct method is expressed as: η= amount of the energy absorbed by the working fluid x 100% energy input The Indirect method is expressed as: sum of energy losses x 100% η = 1 − energy input Page 21 The sum of energy losses considered are: a.) the amount of energy lost in the flue gas b.) the amount of energy lost due to CO in the flue gas c.) the amount of energy lost due to unburned fuel in ash d.) the conduction and convection losses from the boiler structure The overall energy balance for a boiler may be written from the first law of thermodynamics as: Energy input = Energy to working fluid + Energy losses in the flue gas + unaccounted losses Where Unaccounted losses = radiation loss from boiler structure + conduction losses + energy loss to the floor of the boiler house Therefore from first law of thermodynamics: Energy input = Energy to steam + Energy loss in the flue gases + unaccounted loss Divide by the “Energy input” 1= Energy to steam Energy loss in the flue gases unaccounted loss + + Energy input Energy input Energy input Rearranging and neglecting the unaccounted energy loss (usually the unaccounted loss contributes only to around 4% of the total energy input): Energy loss in the flue gases Energy to steam ≈ 1− Energy input Energy input (Direct Method) 1.9. (Indirect Method) Combustion Air The oxygen required in any combustion processes is supplied by the atmospheric air and the oxygen within the fuel itself. As shown in Table 3, biomass has a substantial amount of oxygen within the fuel therefore requires lesser amount of oxygen from the atmosphere. Atmospheric air (as shown in Table 8) has approximately 78% N2 and 21% O2. However, for simplicity in combustion calculation, N2 is taken as 79% and O2 is taken as 21%. With this assumption, the molar ratio of the nitrogen to oxygen is 0.79/0.21 = 3.76. Thus every mole of oxygen supplied from air is always accompanied by 3.76 moles of nitrogen. Also for calculation simplicity, nitrogen in the combustion process does not undergo chemical reaction. (i.e. nitrogen is inert). In most combustion calculation, the parameters that are frequently used to quantify the amounts of fuel and air in a particular combustion process are the air-fuel ratio, theoretical or stoichiometric amount of air and percent excess air. Page 22 Table 8: Typical composition of dry air Components Mole Fraction (%) Nitrogen (N2) 78.08 Oxygen (O2) 20.95 Argon (Ar) 0.93 Carbon Dioxide (CO2) 0.03 Others (Neon, Helium, Methane etc…) 0.01 The air-fuel ratio can be written on a molar basis (moles of air / moles of fuel) or on a mass basis (kg of air / kg of fuel). Conversion between these values is accomplished by using the molecular weight of air (MWair) and fuel (MWfuel). Air - Fuel Ratio (AF) = (moles of air)(MWair ) mass of air = mass of fuel (moles of fuel)(MWfuel ) The molecular mass of air is normally taken as 28.84 (29.0), while the molecular mass of biomass may be calculated from the Ultimate Analysis of the fuel. The minimum amount of air necessary to supply sufficient amount of oxygen to complete the combustion process is called the theoretical or stoichiometric amount of air. To calculate the stoichiometric amount of air, the following global reactions are considered: C + O2 → CO2 2H + ½ O2 → H2O S + O2 → SO2 The products of combustion would then consist only of carbon dioxide, sulphur dioxide, water, nitrogen from air and fuel. (nb: there will be no free oxygen appearing in the combustion product). Note that 1 kmol of O2 is required to burn 1 kmol of carbon atom, ½ kmole of O2 is required to burn 2 kmol of H atoms and 1 kmol of O2 is required to burn 1 kmol of sulphur atom. It should be noted that oxygen in the fuel is used first before oxygen in air is used. Thus the theoretical amount of air is calculated as: stoichiometric amount of O2 = kmol C + ¼ kmol H + kmol S – ½ kmol O in the fuel Likewise, (stoichiometric amount of O2)(0.21)-1 = stoichiometric amount of air Finally, the percent excess air is defined as follows: Percent Excess Air = amount of air supplied x100% stoichiometric amount of air Page 23 Illustrative Example (#3): Determine the air to fuel ratio on mass basis for the complete combustion of woodchips with the following ultimate analysis of woodchips: Carbon 48.6% Hydrogen 5.2% Oxygen 46.0% Nitrogen 0.2% Sulphur 0.0% and burned under the following conditions: a.) only the theoretical amount of air is supplied. b.) When 50% excess air is supplied Solution to Illustrative Example (#3) Calculation Procedure: Calculate the ultimate analysis in dry ash free (daf) basis to as fired basis. Calculate the moles b.) of C, H, O, N and S atoms per kg of fuel. Calculate the c.) stoichiometric amount of air needed per kg of fuel. Basis 100 kg of d.) woodchips and assuming complete combustion. a.) mass of C in woodchip as fired = % C(daf)/100*(100 – moisture – ash) = 48.6/100 * (100 – 12.7 – 0.79) = 42.04 kg C / 100 kg wood Theoretical (stoichiometric) amount of oxygen = kmol of C + ¼ kmol of H – ½ kmol O = 3.503 + 4.50/4 – 2.487/2 = 3.385 kmol of O2 mass of H in woodchip as fired = % H (daf)/100*(100 – moisture – ash) = 5.2/100 * (100 – 12.7 – 0.79) = 4.50 kg H / 100 kg wood Theoretical amount of air on mass basis = 3.385 kmol of O2 / 0.21 * 28.84 = 464.81 kg of air mass of O in woodchip as fired = % O (daf)/100*(100 – moisture – ash) = 46.0/100 * (100 – 12.7 – 0.79) = 39.79 kg O / 100 kg wood moles C (per 100 kg woodchips) = 42.04 / 12 = 3.503 kmol C atoms Solution: Mass of ash (as fired) = % ash/100 x (100 – moisture) = (0.9/100) (100 - 12.7) = 0.79 kg of ash /100 kg wood moles H (per 100 kg woodchips) = 4.50 / 1 = 4.50 kmol H atoms moles O (per 100 kg woodchips) = 39.79 / 16 = 2.487 kmol O atoms a.) air to fuel ratio on mass basis if theoretical amount of air is supplied = 464.81 kg of air / 100 kg of woodchips = 4.65 kg of air / kg of woodchips b.) air to fuel ratio on mass basis if 50% excess air is supplied = 4.65 kg of air / kg of woodchips * 1.5 = 6.97 kg of air / kg of woodchips Challenge Question (i.) Calculate the composition of CO2, O2, N2 and H2O in the flue gas. (assuming that nitrogen in the fuel is inert). 1.10. Products of Combustion Illustrative example #3 assumes that combustion is complete and nitrogen in the fuel is inert. Thus the product of combustion consists only of CO2, H2O, N2 from air, N2 from fuel and SO2. However, in reality, combustion is seldom complete and CO in small quantity is one of the products. Several factors affect the overall combustion process and these include (i.) amount of air supplied, (ii.) degree of mixing between air and fuel, (iii.) temperature and pressure of the combustion surroundings (i.e. furnace), (iv.) overall kinetics of the combustion process. (v.) residence time of the air. For example, when the amount of air supplied is less than theoretical amount required, the products of combustion may include CO2 and CO; and there may also include unburned fuel in the products. Unlike the case of complete combustion process, the products of an incomplete combustion process can only be determined by experiment. Page 24 Most commonly used devices for the experimental determination of the composition of products of combustion are the gas chromatograph, infrared analyser, flame ionisation detector. Measurements obtained from these devices are generally reported as a dry flue gas analysis (ie. moisture is removed from the combustion products). Illustrative Example (#4): (see next page for solution) Woodchips, fed into an underfeed stoker at a rate of 5.8 kg/hr, are burned with dry air. The molar analysis of the flue gas on a dry basis is CO2, 7.3%; CO, 0.3% and O2, 12.7%. Assuming that there are no unburned woodchips in the ash, determine the following: a.) Thermal efficiency of the plant b.) Measured excess air in the flue gas Properties of Woodchips Ultimate Analysis (daf) 48.6 % Carbon 5.2 % Hydrogen 46.0 % Oxygen 0.2 % Nitrogen 0.0 % Sulphur Proximate Analysis and GCV 12.1 % Fixed Carbon (dry) 87.0 % VM (dry) 0.9 % Ash (dry) 12.7 % Moisture 17,756 kJ/kg GCV (as received) Operating Parameters of the boiler Fuel Feed Rate Cooling Water Flow Rate Temperature Water In Temperature Water Out 5.8 kg/hr 7.4 litre/min. 12.6 oC 55.9 oC 1.11. Environmental Issues and Biomass Combustion The control of pollutant emissions is a major factor in the design of modern combustion systems. This is also one of the main driving forces in the development and use of biomass as a renewable energy source. Pollutants of concern related to biomass combustion are: o particulate matter – soot, fly ash, fumes and various aerosols o sulphur oxides – SO3, SO2 o nitrogen oxides –NOx (which consist of NO & NO2), N2O o unburned and partially burned hydrocarbons (PAH, aldehydes, CH4) o dioxin and furan o carbon monoxide o greenhouse gases – CO2, N2O Page 25 Solution to Illustrative Example (#2) Calculation Procedure: a.) Calculate the total energy input to the boiler and the energy absorbed by the cooling water. b.) Calculate the stoichiometric amount of air required for combustion. c.) Calculate the total amount of DFG per 100 kg of fuel. d.) Calculate the amount of O2, CO in the DFG per 100 kg of fuel. e.) Calculate the amount of O2 required to burn the CO in DFG completely to CO2. f.) Basis 100 kg of woodchips and assuming that no unburned fuel in ash. Solution: Thermal Efficiency of the plant (direct) amount of energy absorbed by CW = amount of energy input 22.34 kW * 100% = 28.60 kW = 78.11% Mass of ash (as fired) = % ash/100 x (100 – moisture) = (0.9/100) (100 - 12.7) = 0.79 kg of ash /100 kg wood mass of C in woodchip as fired = % C(daf)/100*(100 – moisture – ash) = 48.6/100 * (100 – 12.7 – 0.79) = 42.04 kg C / 100 kg wood Amount of energy input = (fuel flow rate ) (fuel’s GCV) kg 1 hr kJ = 5.8 17756 hr 3600 s kg mass of H in woodchip as fired = % H (daf)/100*(100 – moisture – ash) = 5.2/100 * (100 – 12.7 – 0.79) = 4.50 kg H / 100 kg wood = 28.60 kW mass of O in woodchip as fired = % O (daf)/100*(100 – moisture – ash) = 46.0/100 * (100 – 12.7 – 0.79) = 39.79 kg O / 100 kg wood Mass flow rate of cooling water 3 7.4 L 0.001 m min. L. 1000 kg = m3 60 s . min. = 0.1233 kg/s Amount of energy absorbed by water = (water flow rate ) (Cp H2O ) (Tout – Tin) 0.123 kg 4.184 kJ (55.9 − 12.6 )o C = o s kg C moles C (per 100 kg woodchips) = 42.04 / 12 = 3.503 kmol C atoms moles H (per 100 kg woodchips) = 4.50 / 1 = 4.50 kmol H atoms Theoretical amount of oxygen = moles C + ¼ moles H – ½ moles O = 3.503 + 4.50/4 – 2.487/2 = 3.385 kmol of O2 Total Dry Flue Gas (DFG)/100 kg fuel kmol of C in the fuel per 100 kg fuel = kmol of C in DFG kmol DFG 3.503 kmol C per 100 kg fuel = (0.3 + 7.3) kmol C 100 kmol DFG = 46.09 kmol DFG per 100 kg fuel moles of O2 in DFG per 100 kg fuel = (0.127)(46.09) = 5.854 kmol of O2 per 100 kg fuel moles of CO in DFG per 100 kg fuel = (0.003)(46.09) = 0.138 kmol of CO per 100 kg fuel moles of O2 required to burn CO = 0.138 kmol / 2 = 0.069 kmol O2 per 100 kg fuel Measured excess air in flue gas amt. of O 2 - amt. O 2 req. to burn CO = theoretical amt. of O 2 (5.854 − 0.069)* 100% = 3.385 = 170.9 % moles O (per 100 kg woodchips) = 39.79 / 16 = 2.487 kmol O atoms = 22.34 kW 1.11.1. Effects of Pollutants Pollutants can be classified as primary or secondary. Priimary pollutants are emitted directly from source while secondary pollutants are formed via reaction with the primary pollutants in the atmosphere. Pollutants primarily affect the environment and human health. Seinfield (1986) indentify four principal effects of air pollutants in the troposphere: o Altered properties of the atmosphere and precipitation o Harm to vegetation o Soiling and deterioration of materials o Potential increase of morbidity and mortality in humans Page 26 1.11.2. Quantification of Emissions Emission levels are expressed in many ways, which can make comparisons difficult and ambiguous. Some examples of these expressions are pounds per million BTUs (lb/million BTU), gram per kilojoules (g/kJ), parts per million (volume basis) at a specific oxygen level (ppm @ certain % of O2) and many others. Concentrations, corrected to a particular level of O2 in the products stream are frequently used in the literature and in practice. The purpose of correcting to a specific O2 level is to remove the effect of various degree of dilution so that a true comparison of emission levels can be made. To correct a concentration measured at a certain level of O2 the following equation can be used: 21% − %O 2[desired level] χ [corrected to a desired O2 level] = χ [at a given O2 level] 21% − %O 2[given level] Illustrative Example (#5): 75 ppm NOx was measured in an exhaust stream containing 1.5% O2. What is the reported NOx value when reference O2 level is .set at 6%. Solution to Illustrative Example (#5): To correct the NOx concentration from a level of 1.5% O2 to a level of 6% O2 the following formula is: 21% − %O 2[desired level] χ [corrected to a desired O2 level] = χ [at a given O2 level] 21% − %O 2[given level] 21% − 6% = 75 ppm @ 1.5% O 2 21% − 1.5% = 57.7 ppm @ 6% O2 1.11.3. Nitrogen Oxides Nitrogen oxides are one of the many pollutants that cause the acid rain. NO and NO2 are the most common oxides of nitrogen produced during combustion. Both are usually lumped together and reported as NOx. Oxides of nitrogen are formed through several mechanisms. Bowman (1992) classifies these into the following three categories: o Thermal NO o Prompt NO o Fuel NO Page 27 The Thermal NO mechanism involves the reaction between atmospheric nitrogen and atmospheric oxygen to form NO. This overall reaction is described by the extended Zeldovich mechanism. It is noted that this mechanisms is highly dependent on temperature and slightly dependent on the O2 concentration. This route of NO formation is only significant when the gas temperature is greater than 1500K (Purvis et al, 2000). The Prompt NO mechanism involves the reaction of hydrocarbon fragments with molecular nitrogen. In this mechanism, NO is formed more rapidly than the thermal NO. Prompt NO is formed either by one of the following mechanisms: o Fenimore CN and HCN pathways. o N2O intermediate route o As a result of superequilibrium concentration of O and OH radicals in conjunction with the extended Zeldovich mechanisms The Fuel NO mechanism involves the formation of NO from fuel bound nitrogen. This is the most significant source of oxides of nitrogen during the combustion of biomass. Generally, NOx from bound nitrogen evolves in two pathway namely: NOx released during devolatilisation as HCN and NH3 species and the NOx released from the bound nitrogen in the char fraction during combustion. Figure 19 shows a simplified mechanism of fuel nitrogen conversion to NO. Figure 20 presents a more detailed nitrogen oxide formation and reduction pathway during biomass combustion. NO Roxid Fuel N HCN O NH O2 RH Rred. NH2 NO N2 NH3 Figure 19: Simplified chemistry for fuel nitrogen conversion in combustion processes (Nimmo et al, 1991). Figure 20: Simplified reaction pathway of the fuel nitrogen during biomass combustion (main path indicated by thick arrows) [Nussbaumer, 1997]. Page 28 NOx emissions can be reduced in many ways. Figure 21 shows various strategies employed to abate NOx emissions. Most of these technologies have been applied in coal combustion. These technologies have also been applied to biomass combustion. Combustion Modification Staged Combustion Reburn Technology Low NOx burner Post Combustion Control Temperature Reduction Selective NonCatalytic Reduction Selective Catalytic Reduction Figure 21: NOx control technologies applied to solid fuel combustion (Adapted from Turns, 1998) Staged combustion: In this method of NOx control, operation of existing burners are applied in such a way to create a rich-lean stage of combustion. Normally this method is applied to suspension burning. In fixed bed combustion, staged combustion is achieved by reducing the air passing through the grate and increasing the amount of secondary or overfire air to achieve rich-lean burning. Reburn Technology: This method is a type of staged combustion. In this method, about 15% of the total fuel is introduced downstream of the main fuel lean combustion zone. Within the reburning zone, NO is reduced via reactions of HCN species with hydrocarbons and hydrocarbon intermediates. Additional air is supplied after the reburning zone to provide the final burnout of the reburn fuel. Low NOx burner: This is a burner designed for low NOx emission by employing fuel or air staging within the flame. Fuel staging creates a sequential lean-rich combustion process while air staging creates a rich-lean process. Temperature Reduction: In this method, the main objective is to reduce the contribution of the thermal NO mechnism. Examples of the reducing temperature technique are flue gas recirculation, water injection and reduced air pre-heating. Selective non-catalytic reduction (SNCR): In this post-combustion control technology, a nitrogen containing additive, either ammonia, urea or cyanuric acid, is injected and mixed with flue gases to effect chemical reduction of NO to N2 without the aid of a catalyst. Temperature is a critical variable and operation within a relatively narrow range of temperatures is necessary to achieve significant NO reduction. Selective catalytic reduction (SCR): In this technique, a catalyst is used in conjunction with ammonia injection to reduce NO to N2. The temperature window for effective reduction depends upon the catalyst used but is contained within the range of about 480K. to 780K (Turns, 1998). The advantage of SCR over SNCR is that greater NOx reduction is possible when operating temperature is lower. However, the disadvantage is cost. Page 29 Reference Antal, M.J. Jr.. (1982). Biomass Pyrolysis: A Review of the Literature: Part I - Carbohydrate Pyrolysis. in Advances in Solar Energy. (Boer, K.W. and Duffie, J.A.. ed.). Vol. 1, pp. 61-111. American Solar Energy Society. Borman, G.L. and Ragland, K.W.. (1998). Combustion Engineering. WCB-McGraw-Hill Inc.. ISBN 0-07-006567-5. Bowman, C.T.. (1992). Control of Combustion Generated Nitrogen Oxide Emissions. in 24th International Symposium on Combustion. The Combustion Institute. pp. 859-878. Chen, Y., Charpenay, S., Jensen, A., Wojtowicz, M.A. and Serio, M.A.. (1998). Modelling of Biomass Pyrolysis Kinetics. 27th International Symposium on Combustion. The Combustion Institute. pp. 1327-1334 CRE.. (1995). The CRE Coal Sample Bank: A Users Handbook. (Compiled by: Paul Burchill) CRE Group Ltd.. Di Blasi, C.. (1993). Modeling and Simulation of Combustion Processes of Charring and Non-Charring Solid Fuels. Progress in Energy and Combustion Science. Vol. 19, pp. 71-104. Dupont-Roc, G., Khor, A. and Anastasi, C.. (1996). The Evolution of the World Energy Systems. Shell International Ltd., SIL Shell Centre, London. FAO, United Nation. (1997). Regional Study on Wood Energy Today and Tomorrow in Asia. Field Document # 50, Regional Wood Energy Development Programme (RWEDP),Bangkok. http://www.rwedp.org/shares.html and http://www.rwedp.org/acrobat/fd50.pdf (October, 1997). IEA (2000a). Energy Policies of IEA Countries: Sweden 2000 Review. International Energy Agency, Paris, France. ISBN 92-64-18523-2-2000 IEA (2000b). Key World Energy Statistics from the IEA. International Energy Agency, Paris, France. htttp://www.iea.org/statist/keyworld/keystats.htm. (1 October, 2001). Klass, D.L.. (1998). Biomass for Renewable Energy, Fuels, and Chemicals. Academic Press Inc.. ISBN 0-12-410950-0. Laurendeau, N.M.. (1978). Heterogeneous Kinetics of Coal Char Gasification and Combustion. Progress in Energy and Combustion Science. Vol. 4, pp. 221-270. Nussbaumer, T.. (1997). Primary and Secondary Measures for the Reduction of Nitric Oxide Emissions from Biomass Combustion. in Developments in Thermochemical Biomass Conversion. Vol. 2, pp. 1447-1461. Blackie Academic and Professional. ISBN 0-75-140350-4. Nimmo, W. Hampartsoumian, E. and Williams, A. (1991). Control of NOx Emission by Combustion Air Staging - The Measurement of NH3, HCN, NO and N2O Concentrations in Fuel Oil Flames. Journal of Institute of Energy. Vol. 64, pp. 128-134. Podolski, W.F., Miller, S.A., Schmalzer, D.K., Fonseca, A.G., Conrad, V., Lowenhaupt, D.E., Bacha, J.D., Rath, L.K., Loh, H., Klunder, E.B., Mc Ilvried, H.G. III.. (1997). Energy Re- sources, Conversion and Utilization. in Perry’s Chemical Engineering Handbook, 7th Edition. (Perry, R.H., Green, D.W. and Maloney, J.O.. ed.) pp. 27-1 to 27-60. McGraw Hill, London. ISBN 0-07-049841-5. Purvis, M.R.I., Tadulan, E.L. and Tariq, A.S.. (2000). NOx Emissions from the Underfeed Combustion of Coal and Biomass. Journal of the Institute of Energy. Vol. 73, pp. 70-77. Rossi, A. (1984). Fuel Characteristics of Wood and Non-Wood Biomass Fuel. in Progress in Biomass Conversion. (Soltes, J and Lin, S.C.K. ed.). Academic Press Inc. Vol. 5, pp. 69-99. ISBN: 0-12-53905-5. Seinfield, J.H.. (1986). Atmospheric Chemistry and Physics of Air Pollution. John Wiley and Sons. ASIN 0471828572. Siau, J.F. (1984). Transport Processes in Wood. Springer-Verlag. ISBN 0-38-712574-4. Solomon, P.R., Serio, M.A. and Suuberg, E.M.. (1992). Coal Pyrolysis: Experiments, Kinetic Rates and Mechanisms. Progress in Energy and Combustion Science. Vol. 18, pp. 133-220. Tsoumis, G. (1991). Science and Technology of Wood: Structure, Properties, Utilization. Van Nostrand Reinhold. ISBN 0-44-223985-8. Turns, S.R.. (1996). An Introduction to Combustion: Concepts and Applications. McGrawHill Inc.. ISBN 0-07-911812-7. Wender, I., Heredy, L.A., Neuworth, M.B. and Dryden, I.G.C.. (1981). Chemical Reactions and the Constitution of Coal. in Chemistry of Coal Utilization: 2nd Supplementary Volume. (Elliott, M.A. ed.). pp. 425-522. John Wiley & Sons, Inc.. ISBN 0-47-107726-7. Williams, A., Pourkashanian, M. and Jones, J.M.. (2000). The Combustion of Coal and Some Other Solid Fuels. in 28th International Symposium on Combustion. The Combustion Institute. pp. 2141-2162.