12 DISTILLATION Distillation is one of the most frequently used
advertisement

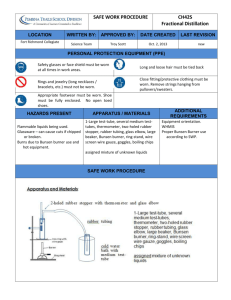
12 DISTILLATION Distillation is one of the most frequently used methods of purification if the products of a reaction are a liquid, if liquid starting materials are required in pure form, or if two or more liquids require separation. Distillation can be defined as a procedure whereby a liquid is converted to vapour, the vapour is removed from the flask and recondensed to a liquid. Distillation may be carried out on liquids containing trace impurities, but more frequently two (or more) miscible liquids are involved and the separation of the mixture into two pure liquids requires fractional distillation (see below). If both liquids exhibit ideal behaviour then a phase diagram may be drawn to describe their behaviour. See Figure 3 on page 13. If a mixture of compound B (boiling point 50°C) and compound A (boiling point 100°C) containing 40% of B is heated, it will boil at a temperature indicated by point Q. The line QR is a tie line and indicates that the vapour in equilibrium with the boiling liquid originally containing 40% B has a different composition of 70% B. (This, of course, differs from the situation for a pure compound in which the liquid and vapour in equilibrium at the boiling point will have the same composition.) If this vapour was removed and "condensed" (liquefied) a simple distillation would have been carried out. The resulting condensed mixture would contain more pure B than the undistilled mixture (undistilled at 40%, distilled 70%). This liquid could be vapourized, the vapour removed and condensed and a mixture with 90% B would be obtained. One more distillation would give almost pure B. Several simple distillations would result in the isolation of pure B from the original 40% mixture; however, this would represent a very inefficient way of separating two such compounds, since some B is lost at every step. 13 14 In practice, a fractional distillation is carried out. Packed fractionating columns are attached to the distilling flask and before the vapour can be removed, as it is in a simple distillation, it hits the cold packing (e.g. in a Hempel column) or the protruding areas (e.g. in a Vigreux column) and is condensed. Hot vapours coming up the column revapourize this liquid and the vapour travels up the column (remember it is now closer to pure B) until it condenses again. This process is repeated several times (i.e. in effect, several simple distillations have occurred) so that the vapour which is finally removed and condensed will likely be pure B, since the vapour of pure B is in equilibrium with the liquid (see Figure 3). This condensing and returning of the liquid to the column is known as refluxing. If no liquid is distilled off and all returns to the column and flask, this is known as total reflux. If all the vapour is removed without any condensation, this is known as "total takeoff". Obviously, neither extreme will give a good distillation and some intermediate ratio of reflux to takeoff is employed. Many pairs of liquids exhibit "non-ideal" behaviour and, although the basic concepts in such distillations are the same, they differ in some important ways. The behaviour of non-ideal liquids falls into two main categories. The two liquids may exhibit either a minimum boiling point (See, for example, Figure 4, page 13) or a maximum boiling point. Taking the former case to illustrate the point, this means that for these compounds there is a mixture, called an azeotropic mixture which behaves essentially as a pure compound, with boiling point 87.2°C (M) which is lower than that of either pure water (100°C) or pure 1-propanol (97.2°C). At this composition the compositions of the vapour and the liquid in equilibrium with one another are identical, whereas in the case of the two ideal liquids A and B (Figure 3), no such point exists. If a mixture of 50% water and 1-propanol is distilled there will be an increase in the concentration of 1-propanol in the vapour phase and there would be a stepwise series of simple distillations similar to the ideal case of A and B. However, when the vapour reaches composition M (71.7% 1-propanol in this case) and is condensed, the resulting liquid will exist in equilibrium with the vapour of the same composition. Therefore, no further change in composition can occur and hence the liquid distilled will be of a constant composition and will continue to distill at 15 87.2°C as long as there is sufficient 1-propanol in the distilling pot to form the azeotrope. In essence, we have an ideal system with pure water as one of the liquids and an azeotropic mixture of 1-propanol and water (with composition 71.7% 1-propanol and 28.3% water) as the other liquid component. Thus it is not possible in this system to obtain pure 1-propanol if you start with a composition with less than 71.7% 1-propanol. When all of the 1-propanol has been used to form the azeotrope there will still be water left in the distilling pot. The distillation temperature will gradually rise and pure water will begin to distill off at 100°C. In summary then, two liquids can behave in one of 3 possible ways when distilled. First, they could behave as ideal liquids with the lower boiling liquid distilling first, followed by the higher boiling liquid and with good separation, as long as there is a difference in boiling points of at least 10°C. Secondly, a minimum boiling point may be observed so that only the azeotrope and one of the two pure liquids may be recovered. Thirdly, a maximum boiling point may be observed. The system you will be investigating today is 1-propanol-water and the physical constants for this system are shown in Figure 4 on page 13. 16 17 Experimental Procedure The following apparatus is used in carrying out the distillation. A round-bottom flask is fitted with a stillhead and a thermometer pocket. A few drops of silicone oil are added to the pocket to improve thermal conductivity and the thermometer inserted. A Liebig condenser is attached to the stillhead and water is circulated through the condenser. An adapter is attached to the condenser and the distillate is collected in an Erlenmeyer flask or other suitable receiver (see Figure 5 on page 16). SAFETY REMINDER: WEAR SAFETY GOGGLES AT ALL TIMES FLAMMABLE LIQUIDS Set up a distillation apparatus as outlined above and shown in Figure 5. Use a 100 mL roundbottom flask and be sure to add boiling chips. Take 50 mL of the 1-propanol-water mixture (50% v/v) supplied and heat the flask so that distillation occurs at the rate of about 15 to 20 drops per minute. Distill into a graduated cylinder and record the temperature and volume at 1 mL intervals during the distillation. Change receivers when the temperature of distillation changes. What is happening here? Continue distilling until only 1 or 2 mL of liquid remains in the distilling flask. Record the total volume of your distillate and the amount remaining in the distilling flask after it has cooled. Comment on the efficiency of this purification method.