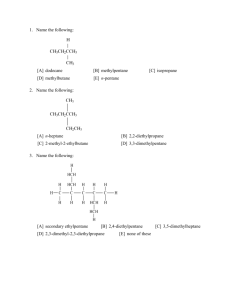
Chemical Nomenclature
advertisement

Chemical Nomenclature: Part III Ternary Inorganic Compounds: IUPAC Nomenclature In addition to these general rules, there are two minor extensions of binary compound nomenclature to ternary compounds, which are those compounds containing three or more different elements. Extension to include mixed salts, which contain different electropositive elements, is fairly simple. Suppose you have a compound such as Na2S, sodium sulfide, in which a potassium replaces a sodium yielding KNaS. This is called potassium sodium sulfide, potassium being named first because it is more electropositive. The compound NaHS would be sodium hydrogen sulfide for the same reason. Those inorganic compounds containing (at least) three elements are called ternary compounds. Unfortunately, there is no single system of nomenclature in use for them. The fully systematic IUPAC method is not used for most of the common compounds. There are two systems of nomenclature for ternary compounds in use other than the IUPAC system. These are the oxygenacid system devised by De Morveau and Lavoisier used for the majority of the common compounds and the system used for coordination compounds. The first of these will be taken up in the following section, but the latter will be deferred to later in senior chemistry. The basic premises of the systematic IUPAC nomenclature of ternary compounds are similar to the ones for binary compounds. Again, the names of the compounds are made up of the names of the elements of which they are composed. Again, the elements are named in the order most metallic to least metallic. However, while the name ending for a binary compound is -ide, the name ending for a more complex inorganic (ternary, quaternary, etc.) compound is -ate. The IUPAC method for naming ternary compounds is to take a binary-type name using two elements, alter the ending to -ate to designate it as a non-binary compound, and then specify the number and type of added atoms as a prefix to the name or names of the element(s) to which they are attached. The number of atoms is given using the Greek prefixes. A few examples suffice to give this method. Example. The compound NaClO4 is named sodium tetraoxochlorate (VII) because the number of oxygens is four, giving tetra oxo; the oxidation state of chlorine should be specified as it is here. Other examples are NaAlCl4, sodium tetrachloroaluminate (III); NaAlCl3F, sodium monofluorotrichloroaluminate (III); Na2SO4, sodium tetraoxosulfate (VI); and Na2SO3, sodium trioxosulfate (IV). Prefixes of other elements used in this way generally end in -o; a few prefixes (aqua, H2O; hydroxo, OH-) give names of simple multi-element groups. This is the system used in naming complex co-ordination structures and as you can see it's cumbersome. 1 Ternary Inorganic Compounds: Oxygen Acids The older oxygen acid method of naming inorganic compounds described in this section is much more commonly used than is the systematic IUPAC method. It differs from the IUPAC method in that the number of oxygens is not given directly (no oxo substituents), and oxidation states are not given. Instead, endings change from -ate, and prefixes are introduced, depending upon the relative numbers of oxygen atoms present. Virtually all of the common ternary compounds have oxygen as one of their components. Lavoisier believed that all acids, and therefore all salts of acids as well, contained oxygen, and his system of nomenclature was unfortunately based on this erroneous idea. The other elements in the compound were therefore named but the oxygen was not. Instead, the amount of oxygen present was denoted by changes in the form of the name. The hypo-ite, ite, ate, per-ate system Take a polyatomic ion. For example ClO3- which is the chlorate ion. It's name ends in "ate". Add an oxygen to it and you get ClO4-. Its name is adjusted to indicate that a new oxygen has been added by putting a prefix "per" on the name. So ClO4- is perchlorate. If we take an oxygen away from the ClO3- ion we get ClO2- the chlorite ion. If we take another oxygen away from ClO2- we get ClO- the hypochlorite ion. Note that the ions did not change their charge. Only the number of oxygens changed and the name changed to reflect it. If you know one of the "ate" or "ite" or even "per-ate" ions then you can manufacture the other three. (The other three may not exist in nature, but you can still create them on paper for naming purposes.) Example: AsO53- is the perarsenate ion. It is "per-ate" so you can't add more oxygen. But you can subtract 3 oxygens one at a time and get three new ions. AsO43- would be arsenate; AsO33- would be arsenite and AsO23- would be hypoarsenite. Try it on your own: Bromate is BrO3- Name and write the other three ions based on it. Compounds can be named using these new ions and following the general rules of combining ions. 2 Example. The sodium salts of the oxyacids of chlorine are named as given below: Remember that Na is a 1+ ion and the chlorine oxy-acids are all 1-. NaClO4, sodium perchlorate (higher oxygen content) NaClO3, sodium chlorate (normal oxygen content) NaClO2, sodium chlorite (lower oxygen content) NaClO, sodium hypochlorite (even lower oxygen content) The compound name changes its ending and/or adds a prefix to denote the relative oxygen content. The prefix per- means higher oxygen content and the prefix hypo- means lower oxygen content. The oxygen content as specified is relative, not absolute; thus NaClO4 with four oxygens is sodium perchlorate while Na2SO4 with four oxygens is sodium sulfate (and so Na2SO3 is sodium sulfite). Unfortunately, chemists rarely use the IUPAC system for the common compounds but retain this older oxygen-acid nomenclature. The IUPAC system is reserved for compounds less well known or of more complex structure. Since the oxygen-acid nomenclature of ternary compounds does not give the absolute number of oxygens involved, this must be derived from experience. Example. Some of the more common sodium salts are named as follows: NaNO2, sodium nitrite; NaNO3, sodium nitrate NaPO3, sodium phosphite; Na3PO4, sodium phosphate Na3AsO3, sodium arsenite; Na3AsO4, sodium arsenate Na2SO3, sodium sulfite; Na2SO4, sodium sulfate Na2CO3, sodium carbonate (no carbonite is known) Na3BO3, sodium borate (no borite is known) Na4SiO4, sodium silicate (no silicite is known) The salts of the halogens fluorine, bromine, and iodine generally follow the pattern of the chlorine salts given earlier. In general, salts of elements follow the same pattern going down a column of the periodic chart - that is, elements of the same group follow the same pattern. While this is generally true it is not always so; phosphorus, arsenic, and antimony follow the same pattern, but nitrogen does not, and silicon and germanium follow the same pattern, but carbon does not. Manganese and ruthenium do not follow these rules. Their negative ions are named as follows: KMnO4 is potassium permanganate, K2MnO4 is potassium manganate; KRuO4 is potassium perruthenate, and K2RuO4 is potassium ruthenate. Although the number of oxygens does not rise in going to these "per" compounds the oxidation state of the central transition metal atom does. The compounds of chromium are unusual in a different way; K2CrO4 is potassium chromate and K2CrO3 is potassium chromite, but K2Cr2O7 is called potassium dichromate. We also name KOCN as potassium cyanate by analogy with the cyanide ion, and K2C2O4 is potassium oxalate because it is the potassium salt of the organic acid called oxalic acid. Stop and do Nomenclature Exercise #7 3 The Binary Acids and Oxyacids Acids are compounds that contain hydrogen. Hydrogen acts like a metal because it tends to lose its one lone electron very easily. It is a gas, not a solid like other metals, only because of its very low atomic mass. The binary acids are those that contain hydrogen and one other element only. Name it normally then drop the "ide" ending and add "ic acid" to get the proper name. Example: HF Hydrogen fluoride Example: HCN Hydrogen cyanide becomes hydrofluoricacid becomes hydrocyanic acid The oxyacid compounds, can either be named as hydrogen salts (Example: HClO4, hydrogen perchlorate), a correct modern system which has been endorsed by IUPAC The older oxygen acid method in which the names of the acid and its salts are related. The two systems of names would give, for the oxyacids of chlorine, the following list (older oxygen acid method first): This is the "hypo-ous, ous, ic, per-ic" system for acids. If a compound should be named "per-ate" change its name to "per-ic acid"; if the compound just ends in "ate" then the name changes to "ic acid". The pattern follows in the examples below. • • • • HClO is named hypochlorous acid OR hydrogen hypochlorite HClO2 is named chlorous acid OR hydrogen chlorite HClO3 is named chloric acid OR hydrogen chlorate HClO4 is named perchloric acid OR hydrogen perchlorate Have you noted the essential difference between naming acids and everything else? The only difference between the names is the replacement of hydrogen -ate by -ic acid and the replacement of hydrogen -ite by -ous acid. Since sulfur and oxygen are similar in their chemistry, sulfur can sometimes replace oxygen in a ternary compound. The replacement of one oxygen with one sulfur is denoted by the additional prefix thio. Multiple replacement, which is rare, is denoted by additional numeric prefixes as dithio or trithio. Example. The compound NaOCN, sodium cyanate, becomes NaSCN, sodium thiocyanate, on replacement; Na2SO4, sodium sulfate, becomes Na2S2O3, sodium thiosulfate. Stop here and do Nomenclature Exercise #8 4 Nomenclature Exercise #7 Creating Formulas and Names for the Oxy-Acid Polyatomic Radicals Using the formulas below come up with the correct IUPAC names. 1. NaNO2 21. NaBrO3 2. Ti(SO3)2 22. Ni(MnO4)3 3. NiPO3 23. AuClO2 4. Ag3AsO4 24. Co(IO3)3 5. Sn(BrO2)2 25. Ba3(PO3)2 6. Be(MnO4)2 26. Mg3AsO4 7. Li2CrO3 27. Zn(ClO3)2 8. KClO4 28. Cd(NO2)2 9. NaClO 29. Ag2SO4 10. Ca(IO4)2 30. LiBrO2 11. Bi(IO)5 31. TlPO3 12. Tl(NO3)3 32. Bi(NO3)5 13. PbSO4 33. Ti(BrO2)4 14. K2SO3 34. Co2(SO3)3 15. Ca3(PO4)2 35. Fe3(AsO3)2 16. Hg3AsO3 36. AlPO4 17. Cr(BrO3)6 37. Sn(IO3)4 18. Li2MnO4 38. Tc(IO)7 19. Tc(ClO3)7 39. Al(IO2)3 20. Ba(IO2)2 40. Pb(CrO3)2 5 Given the names below provide the correct IUPAC formulas. 1. Calcium nitrite 21. Mercurous bromate 2. Iron (II) sulphite 22. Chromium (II) periodate 3. Beryllium phosphate 23. Barium bromite 4. Cobaltous sulphate 24. Lithium perchlorate 5. Tin (II) hypoiodite 25. Bismuthous nitrite 6. Barium phosphate 26. Calcium sulphite 7. Potassium hypochlorite 27. Chromium (VI) nitrate 8. Aurous arsenite 28. Gold (III) sulphate 9. Plumbic chlorate 29. Beryllium arsenate 10. Silver nitrate 30. Aluminum hypochlorite 11. Titanium (IV) phosphite 31. Potassium iodate 12. Potassium chromate 32. Thallium (III) sulphate 13. Cadmium perchlorate 33. Mercuric nitrite 14. Mercury (II) phosphate 34. Cadmium arsenite 15. Chromium (VI) iodite 35. Magnesium bromate 16. Nickel (III) chromite 36. Aluminum chlorite 17. Silver tungstate 37. Strontium tellurite 18. Aluminum bromite 38. Magnesium chlorate 19. Tin( II) arsenate 39. Zinc chlorite 20. Bismuth (V) hypochlorite 40. Thalium (III) iodite 6 Nomenclature Exercise #8 Creating Formulas and Names For the Halogen and Oxy-Acids Using the formulas below come up with the correct IUPAC names. 1. HF 2. HI 3. HCN 4. H3PO3 5. H3AsO3 6. H2SO4 7. H2CrO3 8. HBrO3 9. HNO3 10. HClO3 12. HClO2 11. HIO3 Given the names below provide the correct IUPAC formulas. 1. Hydrochloric acid 2. Hydrobromic acid 3. Phosphoric acid 4. Arsenic acid 5. Carbonic acid 6. Sulphurous acid 7. Chromous acid 8. Cyanic acid 9. Nitrous acid 10. Perchloric acid 11. Acetic acid 12. Iodous acid 7