Chemistry Vocabulary The atom (helium) Atomic number: 2 (2
advertisement

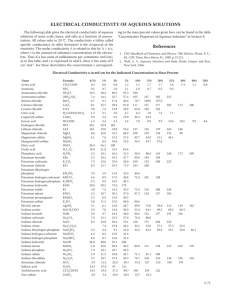
Chemistry Vocabulary The atom (helium) Atomic number: 2 (2 protons) Atomic mass: 4 (2 protons + 2 neutrons). Only one isotope of helium commonly occurs. Number of electrons: 2 Electrons Protons Neutrons A proton has a positive electric charge. An electron has a negative electric charge. The states of matter sublime melt solid (ice) evaporate liquid (water) solidify freeze gas (vapour) condense sublime Elements and compounds The simplest elements Atomic 1 2 3 4 5 6 7 8 9 10 11 12 13 Name hydrogen helium ithium beryllium boron carbon nitrogen oxygen fluorine neon sodium magnesium aluminium Symbol H He Li Be B C N O F Ne Na Mg Al Most requent number valencies 1, –1 1 2 3 4, –4, 2 3, –3, 5, 4, 2, 1 –2 –1 1 2 3 14 15 16 17 18 19 20 silicon phosphorus sulphur chlorine argon Si P S Cl Ar potassium calcium K Ca Elements whose symbols are very different: iron copper silver tin gold lead Fe Cu Ag Sn Au Pb Common anions with their valencies: hydride carbonate nitride nitrite nitrate oxide peroxide hydroxide fluoride phosphate sulfide sulfite sulfate chloride chlorite chlorate H– CO32– N3– NO2– NO3– O2– O22– OH– F– PO43– S2– SO32– SO42– Cl– ClO2– ClO3– 4 3, –3, 5 –2, 6, 4 1, –1, 3, 5, 7 1 2 Example compounds: Sodium hydride Lithium carbonate Boron nitride Sodium nitrite Silver nitrate Zinc oxide Barium peroxide Potassium hydroxide Hydrogen fluoride Tricalcium phosphate Iron (II) sulfide Sodium sulfite Copper (II) sulfate Potassium chloride Sodium chlorite Potassium chlorate NaH Li2CO3 BN NaNO2 AgNO3 ZnO BaO2 KOH HF Ca3(PO4)2 FeS Na2SO3 CuSO4 KCl NaClO2 KClO3 Chemistry Vocabulary Exercises 1. Write the translation of the words. 1 hectogram 2 silicon 3 nanosecond 4 evaporate 5 molar mass 6 isotope 7 hydrogen a…………………………. b…………………………. c…………………………. d…………………………. e…………………………. f…………………………. g…………………………. 2. Complete the definitions with a word from the box. charge compound liquid metal molecule reaction 1 If a substance sublimes it passes from a solid to a gas, without becoming a ...l.i.q..u..i.d..... . 2 A ............... is the smallest structured particle of a substance, with no electric charge. 3 Lead is a soft, very dense, poisonous ................ 4 An element’s electric ............... is given by subtracting the number of electrons from the number of protons. 5 A substance’s valency gives the number of ions that will combine to form a ................. 6 An equation such as H2+Cl2 = HCl represents a chemical ................. 3.Match the names of the compounds with the formulae and the descriptions 1.sodium chloride 2.hydrogen chloride 3.carbon dioxide 4.aluminium oxide 5.nitrous oxide 6.hydrogen peroxide 7.calcium carbonate N2O H2O2 Al2O3 NaCl CaCO3 CO2 HCl alumina, taken from bauxite ore to make aluminium chalk, limestone, and shells a gas which makes hydrochloric acid when dissolved in water ‘laughing gas’, used as an anaesthetic table salt a gas that we breathe out and plants use a watery liquid used for cleaning wounds and bleachin hair 4.Write the English names of these compounds 1 2 3 4 5 6 KNO3 NaOH LiF AuCl3 MgCO 3 H2O ........potassium nitrate.. ....................................... ....................................... ....................................... ....................................... .......................................