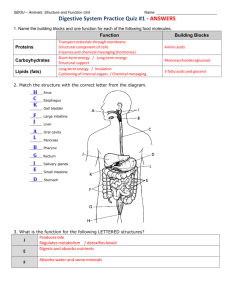
Lara Slough
advertisement

Histology of the Digestive System of Scyliorhinus retifer, the Chain Catshark By Lara Slough Advisor: Dr. John Morrissey Abstract I prepared a histological description of Scyliorhinus retifer, the chain catshark, to show that the digestive tissues resemble those of other vertebrates. After taking ten tissue samples, I prepared them for observation. I observed my slides using light microscopy and took photomicrographs. My results show similarities between the digestive tissues of Scyliorhinus retifer and those of other vertebrates. These results indicate similar function of the digestive systems as well as a common ancestor. Introduction During the summer of 2008, I conducted a research project about the digestive system of Scyliorhinus retifer, the chain catshark. This small, deep-dwelling elasmobranch inhabits the waters of the outer continental shelf and slope from Nova Scotia to Nicaragua. Elasmobranchs, which include sharks and rays, are in a sub-class of the class Chondrichthyes that have yet to be studied extensively. The chain catshark reaches approximately 45cm in length as an adult. Seeking an optimal temperature of 1112°C, it is found at average depths of 70-550m. Little is known about its biology and habits. (Compagno, 1984) I wondered if the scarcity of food in the deep sea might have led to adaptations in the digestive system of this shark. This was certainly a possibility. However, because all vertebrates with rare exception have similarly functioning digestive systems, I expected it to function in basically the same way as in other vertebrates. I studied histology to confirm or deny similarities. There have only been a couple of publications regarding the basic range and distribution of this species, and there is some information regarding its reproduction, but no studies about its digestive system and histology have been published to date. (J. Morrissey, pers.com) Histology is the study of tissues, groups of cells with similar structure that function together as a unit. If the tissues appear similar, the function is also similar. (Gartner, 2001) My hypothesis was that the digestive tissues of Scyliorhinus retifer would appear similar to the digestive tissues of other vertebrates. 1 I compared my results to other vertebrates to prove my hypothesis. Because human beings have already been compared histologically to numerous vertebrates, I used the human digestive system as a standard for comparison. (Derrickson, 2006) I also compared my results to primitive fishes, such as sturgeons and elasmobranchs. Humans and sharks have similar digestive systems, the basic function of which is to break down food for absorption. The systems appear somewhat similar upon macroscopic observation of the organs. The major organs involved include the mouth, esophagus, stomach, intestine, and rectum. There are some differences although the function is the same. The stomach and intestine differ slightly in humans and sharks. The stomach serves to break down larger pieces of food into smaller pieces of food in both humans and sharks. The intestine of the shark is adapted to the stream-lined body shape of the shark. The spiral valve results in a compact design as well as providing maximum surface area for nutrient absorption. (Holmgren, 1999) In the human, the intestine is long and coiled; the small and large intestine have a combined length of nearly 9 meters. This coiled design also provides maximum surface area for nutrient absorption, but is more appropriately shaped for the human organism (Derrikson, 2006). Food follows a specific path through the digestive tract. In the human, the food enters the oral cavity, travels through the esophagus, and into the cardiac stomach. This is the first section of the human stomach, and is heavily muscled to break larger pieces of food into smaller pieces of food. The muscles of the stomach create a churning motion that breaks up the food. Then the food travels through the second section of the stomach, the fundus. Here, the smaller pieces of food are broken down into even smaller pieces of food. The third section of the stomach is called the pyloric stomach, in which the food is broken down into the smallest particles possible in the stomach. The pyloric stomach opens into the intestine via the pyloric sphincter, the muscular gateway between the pyloric stomach and the intestine. The food travels through the long coils of the intestines, where the nutrients are absorbed, and the remaining waste is excreted through the rectum and out the anal opening. (Derrickson, 2006) 2 The path food follows in the shark is similar to the path food follows in the human. In the shark, the food enters the oral cavity, travels through the esophagus and into the cardiac stomach. Sharks have a two-section stomach (Figure 1a), whereas humans have a three-section stomach. In the cardiac stomach, as in humans, larger pieces of food are broken down into smaller pieces of food. The food enters the second part of the stomach, the pyloric stomach, where smaller pieces of food are broken down into even smaller pieces of food. After the pyloric stomach has sufficiently broken down the food, it passes through the pyloric sphincter. The sphincter contracts to keep insufficiently processed food from passing through and relaxes to allow sufficiently processed food to pass through into the intestine. As the food travels through the intestine, nutrients are absorbed. Because sharks have a spiral intestine, nutrients are absorbed while traveling through an encased spiral valve (Figure 1b) rather than long coils as in the human. The leftover waste travels through the rectum and out the anal opening. (Holmgren, 1999) Figure 1a. The Shark Digestive System. (Holmgren, 1999) Figure 1b. Inside the Spiral Intestine. (Holmgren, 1999) I studied histology because histology accounts for apparent differences such as the human intestine and the shark intestine, which could be misleading. Histology, which is the study of tissues, provides a description of an organism on a fundamental level. 3 Similarities at this level are most important because they prove similarities in function. There is a hierarchy to the structure of an organism. Individual cells make up tissues, tissues make up organs, organs make up organ systems, and organ systems make up the organism. The type of tissue indicates the function of the tissue, the function of the organ, the function of the organ system, and the function of the organism. There are four types of tissue: epithelial, connective, muscle, and nervous. Epithelial tissue protects, secretes, absorbs, lines hollow organs, and also forms glands. Connective tissue provides a supportive framework for the body. Muscle tissue allows the body to move. Nervous tissue controls all functions of the body. The tissues of the human intestine and the shark intestine are histologically similar. Therefore, they are functionally similar as well. I set out to prove that the chain catshark was histologically similar to other vertebrates. The focal tissues of my project, epithelial and muscular, are most prevalent within the digestive system, although all four tissue types are present. There are several different types of epithelial tissue, each descriptively named based on the number of cell layers and cell shape. There are three types of muscle. Smooth muscle is involuntarily controlled and is found in the digestive tract. Skeletal muscle is voluntarily controlled and is found, for example, in the biceps. Cardiac muscle is involuntarily controlled and is found in the heart. (Carneiro, 2005) Methods To compare the digestive system tissues of the chain catshark to those of other vertebrates, I completed several preparatory steps. After the digestive system had been removed from the dissection specimen, I took tissue samples from certain areas along the digestive tract, collecting ten samples in all (Figure 2). 4 1. Oral Cavity Dorsal 3. Esophagus 8. Spiral Intestine A 9. Spiral Intestine B 10. Rectum 7. Pyloric Sphincter 6. Pyloric Stomach 2. Oral Cavity Ventral 4. Cardiac Stomach A 5. Cardiac Stomach B Figure 2. Tissue Sample Locations 1-10 (in numerical order following food path). After collecting the tissue samples, I prepared them for observation. I dehydrated the tissue samples by soaking them in ethanol, which is water soluble, and Safe-clear, a tissue clearing agent. This process removes water from the sample, resulting in the space normally taken up by water, about 80% of the tissue (Derrickson, 2006), being filled with something more conducive to sectioning. I then embedded the tissue samples in paraffin wax, which creates a hardened sample that is ideal for sectioning. I left the samples in the refrigerator overnight to make sure they were thoroughly hardened. To ensure the samples were hard enough to section, yet soft enough to avoid splintering, I removed the samples from the refrigerator 30 minutes before sectioning. Using a microtome set to 10 micrometers, I sectioned each sample. I placed the ribbon of wax with embedded tissue into a water bath and let it float to flatten out. The water bath was 45°C, to soften yet not melt the wax. After the wax had softened, I submerged a microscope slide in the water and caught the ribbon on the microscope slide as I pulled it 5 up and out of the water. I placed the slides on a slide warmer, also 45°C, to dry. I made at least three slides from each tissue sample. After the slides were dry, I began the staining process. Staining requires soaking the slides in a series of chemicals that ultimately darken the tissue sample and stain it purple, making the tissues visible. I loaded the slides into a tray and submerged them in the appropriate sequence of chemicals for the appropriate amounts of time. I had to break down the paraffin and re-hydrate the tissues so that the dye could sufficiently permeate the tissues to make them visible. This required reversing the procedure I had followed earlier in order to dehydrate the tissue samples. I soaked the slides in Safe-clear and ethanol, the dyes hematoxylin and eosin, and associated chemicals. After the staining process was complete, I let the slides dry, applied cover-slips, and let them dry a final time. They were finally ready to observe under the microscope. I observed my slides using light microscopy, noting muscle and epithelial layers. I took numerous photomicrographs, taking care to capture a good image of each of the tissue samples at different magnifications, finding that 4x, 10x, and 40x magnifications gave the best results. These photomicrographs provided me with a histological description of the chain catshark which I compared to humans as well as primitive fishes. I compared tissues and layers present, noting similarities. Results The photomicrographs I took of the digestive tissues of Scyliorhinus retifer appear similar to photomicrographs taken of the digestive tissues of other vertebrates. White spaces inside the organs, epithelial linings, muscle layers, and white spaces outside the organs are all present. Figure 3a-f shows my results from the cardiac stomach, Figure 4a-f shows my results from the pyloric stomach and the pyloric sphincter, and Figure 5a-f shows my results from the spiral intestine. My results indicate that the basic histology of the gut wall of the chain catshark is similar to that of other vertebrates. Following the path of food, I examined my slides and took photomicrographs, focusing on the stomach and the intestine. I took photomicrographs from two areas of the 6 cardiac stomach to observe any structural changes traveling father down the digestive tract. My photomicrographs of the cardiac stomach proximal to the esophagus (Figure 3a, 3c, 3e, 3f) show the layered structure of the stomach wall. There is white space indicating the inside of the organ, mucosa (a lining composed of simple columnar epithelium), sub-mucosa (loose connective tissue that supports the mucosa), dual muscle layers (interior: circular muscle, CM, exterior: longitudinal muscle, LM), and white space indicating the outside of the organ (Figure 3a). Dense surface cells for secretion are visible upon closer examination (Figure 3c). There are dense dual muscle layers. The layers formed are thick and distinct (Figure 3e, 3f). My photomicrographs of the cardiac stomach distal to the esophagus (Figure 3b, 3d) show the slight differences in structure in this area of the stomach. There is white space indicating the inside of the organ, mucosa, submucosa, circular muscle, longitudinal muscle, and white space indicating the outside of the organ (Figure 3b). Notice that while the same layers are present as in the proximal stomach, yet they are neither as dense nor clearly defined (Figure 3d). Mucosa White Space: Inside Organ CM & LM CM & LM White Space: Inside Organ Mucosa Surface Cells for Secretion White Space: Inside Organ Mucosa Submucosa White Space: Outside Organ Submucosa 3a. Cardiac Stomach A. 3b. Cardiac Stomach B. 3c. Cardiac Stomach A. 4x magnification 4x magnification 10x magnification Submucosa Mucosa 7 Dual Muscle Layers CM & LM Mucosa Close-up of Circular Muscle Submucosa Close-up of Longitudinal Muscle White Space: Outside Organ 3d. Cardiac Stomach B. 10x magnification 3e. Cardiac Stomach A. 10x magnification 3f. Cardiac Stomach A. 40x magnification Figure 3a-f. Photomicrographs of the Cardiac Stomach of Scyliorhinus retifer. The pyloric stomach has white space indicating the inside of the organ, mucosa, submucosa, circular muscle, longitudinal muscle, and white space indicating the outside of the organ (Figure 4a). The mucosa is simple columnar epithelium, and the submucosa is loose connective tissue, as in the cardiac stomach. My photomicrographs clearly show these layers at higher magnifications (Figure 4b, 4c). The layers appear different than the layers in the cardiac stomach (Figure 3a, 3b). There is a clear distinction between the basal membrane of the epithelial layer and the inner layer of submucosa (Figure 4c). The white space indicating the inside of the organ, the circular shape of the pyloric sphincter, and the white space indicating the outside of the organ are visible in the cross-section (Figure 4d). The smooth muscle that makes up this sphincter is shown at higher magnifications as well, showing the density of the fibers (Figure 4e, 4f). Submucosa Close-up of Basal Membrane 8 Mucosa White Space: Inside Organ Mucosa CM & LM Submucosa Close-up of Submucosa CM & LM White Space: Outside Organ 4a. Pyloric Stomach. 4b. Pyloric Stomach. 4x magnification 10x magnification 4c. Pyloric Stomach. 40x magnification White Space: Inside Organ Smooth Muscle Tissue Muscular Ring Muscular Ring Close-up of Muscular Ring White Space: Outside Organ 4d. Pyloric Sphincter. 4x magnification 4e. Pyloric Sphincter. 10x magnification 4f. Pyloric Sphincter. 40x magnification Figure 4a-f. Photomicrographs of the Pyloric Stomach and the Pyloric Sphincter of Scyliorhinus retifer. I compared the structure of the intestine at different places along the digestive tract to see if the structure changed as I had done with the cardiac stomach. I took photomicrographs of two samples from the spiral intestine, one proximal to the pyloric 9 sphincter (Figure 5a, 5b) and one distal to the pyloric sphincter (Figure 5c, 5d, 5e, 5f). There is white space indicating the inside of the organ, an epithelial layer lining the interior of the organ with pockets for absorption, a layer of muscles lining the exterior of the organ, and white space indicating the outside of the organ (Figure 5a, 5c). The structure of the intestinal wall is similar to the stomach wall, mucosa, submucosa, circular muscle, and longitudinal muscle. The spiral valve is visible in cross-section (Figure 5c). The photomicrographs clearly show the contrast between the larger pockets for absorption proximal to the pyloric sphincter (Figure 5a), and the smaller pockets for absorption distal to the pyloric sphincter (Figure 5b). The epithelial lining and outer muscle layers are not easily distinguishable in the proximal sample (Figure 5a). The epithelial lining is more easily distinguished from the outer muscle layer in the distal sample (Figure 5d, 5e). The distal sample also shows surface cells for absorption (Figure 5d, 5f). White Space: Outside Organ CM & LM CloseUp of White Space: Inside Organ Closeup of Inner Lining CM & LM Smaller Pockets for Absorption 10 Larger pockets for absorption Spiral Valve White Space: Inside Organ White Space: Inside Organ 5a. Spiral Intestine A. 4x magnification White Space: Inside Organ CM & LM White Space: Outside Organ 5b. Spiral Intestine A. 40x magnification White Space: Outside Organ Closeup of CM & LM 5c. Spiral Intestine B. 4x magnification White Space: Inside Organ Surface Absorptive Cell White Space: Inside Organ Close-up of Surface Absorptive Cell 5d. Spiral Intestine B. 10x magnification 5e. Spiral Intestine B. 40x magnification 5f. Spiral Intestine B. 40x magnification Figure 5a-f. Photomicrographs of the Spiral Intestine of Scyliorhinus retifer. Discussion The photomicrographs I took showed muscle layers and epithelial layers matching the general structure found in other vertebrates in all major digestive organs, though I focused on the stomach and intestine. These two organs are responsible for the majority of the digestive process, and so similarities in these organs are most important. 11 Mechanical digestion occurs in the stomach, and absorption occurs in the intestine. The gut wall of Scyliorhinus retifer has the same basic structure as human: epithelial layers lining the inside and muscle layers lining the outside. The epithelial layers in the stomach contain cells that secrete acids, enzymes, and mucus. The acids and enzymes aid in digestion and the mucus protects the underlying tissue from damage. The mucosa of the gut wall is simple columnar epithelium, which is a single layer of column-shaped epithelial cells. It is supported by the submucosa, which is loose connective tissue, and dual muscle layers beneath. The dual muscle layers, the circular muscle and the longitudinal muscle, contract perpendicular to each other to create the churning motion that moves food along the digestive tract and breaks up larger pieces into smaller pieces. Gut wall layers are consistent throughout the digestive tract, differing slightly depending on location and function. The mucosa of the stomach is specialized for secretion, while the mucosa of the intestine is specialized for absorption. The layers also vary in density and thickness. (Cormack, 1993) In my photomicrographs, the differences in layers are visible along the digestive tract as the organ function changes, depending on the stage of digestion. I observed the organs in order along the digestive tract: the cardiac stomach A (Figure 3a) and B (Figure 3b), the pyloric stomach (Figure 4a), the pyloric sphincter (Figure 4d), and the spiral intestine A (Figure 5a) and B (Figure 5b). The distinction between the layers is most apparent in the cardiac stomach (Figure 3e, 3f). The histological changes that occur along the digestive tract to compensate for the smaller particles of food are apparent in my results. The first part of the intestine that the food passes through, just after the pyloric sphincter, has larger areas for absorption which is consistent with the larger pieces of food that would be found there. By the time the food reaches the latter part of the intestine, it has been broken down into much smaller particles, and the structure reflects this change in size. (Derrickson, 2006) In the case of the spiral intestine, there are much smaller and more numerous pockets for absorption farther down the intestine. (Holmgren, 1999) This progression is clearly visible in my photomicrographs. The contrast between the proximal (Figure 5a) and the distal (Figure 5c) intestine is striking. 12 Conclusion I observed macroscopic and microscopic similarities between the digestive organs of the chain catshark and the digestive organs of other vertebrates. To prove my hypothesis, I compared my results to photomicrographs taken of tissue samples taken from humans and primitive fishes. Of course, I took many more photomicrographs than I was able to include in my figures for this paper. I considered all of these when making a histological comparison between Scyliorhinus retifer and another vertebrate. When comparing my results to the human, I found gut wall structure was similar. (Cormack, 1993) When comparing my results to the shovelnose sturgeon, Scaphirhynchus platorynchus, I found similarities in the dorsal and ventral oral cavity, including taste buds and cartilage. I also found similarities in the spiral intestine and other organs. (Weisel, 1979) The similar structures of the esophagus and spiral intestine were especially striking in a shark, the narrownose smooth-hound, Mustelus schmitti. (Codon, 1988) I noticed similarities to a ray as well, the thornback ray, Raja clavata, particularly in the structure of the gut wall and the rectum. (Holmgren, 1999) Similarities in the oral cavity, intestine, and other organs were apparent in freshwater fishes as well, the longnose sucker, Catostomus catostomus, and the squawfish, Ptychocheilus oregonense. (Weisel, 1962) All of these comparisons led me to conclude that these results indicate similar tissues, similar tissue function, and therefore similar digestive organs and digestive systems. Similarities at this basic structural level between seemingly vastly different organisms also indicate a common ancestor and evolution. Basic structural components retained from an ancestor and then built upon by evolution may not be obvious, but can be observed. The shark intestine seems entirely different from the human intestine, but histological comparison shows the same types of cells present in both organs, showing that the shark intestine and the human intestine are the same type of organ made up of the same types of cells, evolved into different shapes more suited to the particular organism. The results support my hypothesis: the digestive tissues of Scyliorhinus retifer appear similar to the digestive tissues of other vertebrates. 13 References “The Biology of Sharks and Rays”. Website. http://www.elasmo-research.org/education/white_shark/digestion.htm Carneiro, Jose. Junqueira, Luiz Carlos. Basic Histology Text and Atlas: 11th Edition. The McGraw-Hill Companies, Inc. New York: 2005. Codon, Stella M. Estecondo, Silvia. Galindez, Elena J. “Estudio Anatamo-Histologico del Tracto Digestivo de Mustelus schmitti (Chondrichthyes, Triakidae)”. Physis (Buenos Aires), Secc. A, 46(110):31-41. 1988. Compagno, L.J.V. FAO species catalogue. Vol. 4. Sharks of the World: An annotated and illustrated catalogue of shark species known to date. Part 2. Carcharhiniformes. FAO fish synop. (125) Vol.4, Pt. 2: 251-655. 1984. Cormack, David H. Essential Histology. J.B. Lippincott Company. Philadelphia: 1993. Derrickson, Bryan. Tortora, Gerard J. Principles of Anatomy an Physiology: 11th Edition. John Wiley and Sons, Inc.: 2006. Gartner, Leslie P. Hiatt, James L. Color Textbook of Histology: Second Edition. W.B. Saunders Company. Philadelphia: 2001. Holmgren, Susanne. Nilsson, Stefan. “Digestive System”. Sharks, Skates, and Rays: The Biology of Elasmobranch Fishes: Edited by William C. Hamlett. Johns Hopkins University Press: 1999. Morrissey, John. Faculty Sponsor. Homepage. Weisel, George F.. “Comparative Study of the Digestive Tract of a Sucker, Catostomus 14 catostomus, and a Predaceous Minnow, Ptychocheilus oregonense.” American Midland Naturalist. Vol. 68, No. 2 (Oct., 1962), p 334-346. University of Notre Dame: 1962. Weisel, George F.. “Histology of the Feeding and Digestive Organs of the Shovelnose Sturgeon, Scaphirhynchus platorynchus”. Copeia, 1979, No. 3: 518-525. The American Society of Ichthyologists and Herpetologists: 1979. 15