Lab: Gas Laws Chemistry Chap 12 Do this on your own paper
advertisement

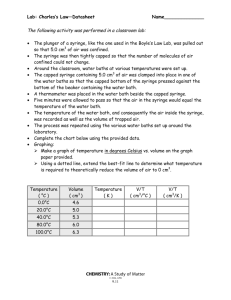
Lab: Gas Laws Chemistry Chap 12 Do this on your own paper Part 1: Charles’ Law: Make a data table like Part 1 Data Table to record data for this step. Make sure you have at least 10 blank rows on this table. Record the current room pressure from the board. Convert to mm Hg and kPa. Learning Target- Given the lab equipment set up, writes and carries out a procedure to measure the change of volume with a change of temperature and graphs the data. Safety: Write the safety needs of this lab. DO NOT LET SYRINGE OR THERMOMETER TOUCH BOTTOM OF GLASS **CAUTION: Thermometers are fragile glass instruments that can break,: minimize glass to glass contact. Consider all glass as hot. Procedures (Augment your procedures with these guidelines) 1. Draw the apparatus (figure C, on right) on your prelab. 2. Remove the end cap of a 60-cm3 syringe and fill the cylinder to a volume of 20 cm3 with air. 3. Replace the cap and secure the syringe within a utility clamp. NOTE: The volume scale should not be obscured by the clamp. 4. 250 mL of water at room temperature to a 400-mL beaker. 5. Lower the Syringe far enough into the water so that at least 20 cm3 of air in the syringe will be below the water surface. Lower the thermometer to a point at which the bulb is about even with, or slightly above, the midpoint of the column of air in the syringe, in order to obtain a temperature that accurately represents that of the contained air. Secure the thermometer in place with a utility clamp placed around the rubber stopper. The syringe and the thermometer should not touch each other, and neither should touch any part of the beaker. 6. Place the hot plate on high and record the initial temperature. 7. For every 10C rise in water temperature, record the gas volume and temperature. Before each measurement, gently push the plunger down a small amount and then release it quickly. Do not let the syringe touch the beaker. 8. Continue until the boiling temperature is reached and take three readings, 30 seconds apart, beyond boiling, to ensure the gas has completely warmed to the temperature of the water. 9. Clean up all materials. Gently add two ice cubes to your hot water (DO NOT SPLASH). Disucuss if your observation indicate a chemical or a physical change. Why do you think this happened? 10. As a lab group, discuss the relationship between temperature and volume (direct, inverse). If confused, consider our discussion of relationships between force, area and pressure. 11. Clean up your desktop. Leave the hot items on the station to cool. Data Analysis : for TASK #1. Clearly label, Show work on calculations, complete sentences with IQIA 1. Graph your data from part 1 on a full piece of graph paper. Put volume (mL) on the y-axis from 0.0 mL to your max. mL. Put temperature (OC) on the x-axis from -300 OC to 100 OC. (you extrapolate your data to absolute zero, - 273 oC) 2. What type of relationship does this graph illustrate between the two variables? Explain. 3. On the Graph, create a best-fit straight line using the points you recorded in question #1. (Ignore the part that is flat, where the syringe didn’t move.) Extrapolate (extend) the line to find the temperature at 0.0 mL volume (This temperature is called absolute zero). Record that temperature number from your graph. Explain why this temperature must be obtained by extrapolation instead of direct observation? 4. Use the kinetic molecular theory to explain how the volume of a gas could reach zero if the temperature were low. 5. What temperature scale has absolute zero as its zero point? 6. Determine the absolute error and percent error of the number you extrapolated from this accepted value (T= -273 OC) Absolute Error = accepted value – experimental value Percent(%) Error = Accepted value – Experimental value x 100 Accepted value