- Wiley Online Library
advertisement

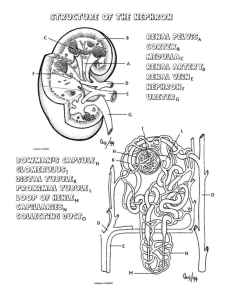
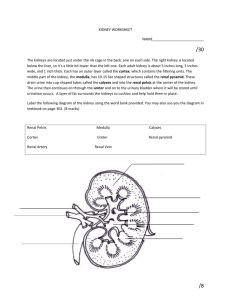
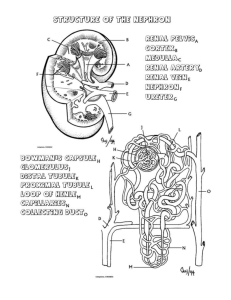
THE ANATOMICAL RECORD 212:33-40 (1985) Structure of the Avian Kidney I. MORTLD, A. BOHLE, AND J.A. CHRISTENSEN Department of Pathology, The Gade Institute, University of Bergen, N-5016 Haukeland Hospital, Norway (Z.M.,J.A.C.)and Institute of Pathology, University of Tubingen, 0-7400 Tubingen, Federal Republic of Germany (A.B.) ABSTRACT The kidneys from 6 domestic fowl were fixed in situ by perfusion from the left ventricle. In the bird there are two types of nephrons. One reptiliantype without Henle’s loop and medullary tissue, and one mammalian-type with Henle’s loop lying in medullary tissue. Serial sections from kidney tissue embedded in plexiglass or in paraffin were used to study the architecture of eight reconstructed reptilian-type nephrons from different cortical levels. All reconstructed nephrons had four major bends, but particularly in the subcapsular nephrons additional bends parallel to the kidney surface were found. There was no loop of Henle, but before entering the collecting duct the distal tubule usually had a very thin-walled segment. No proximal convoluted part was found in the reptilian-type nephrons. The length of the tubules varied between 3,000 pm and 6,000 pm. In the distal tubule a macula densa segment was found in all nephrons of the reptilian and mammalian type. The capillary network between the inter- and intralobular veins was composed of increasingly larger capillaries towards the intralobular vein. Segments of the distal tubule were indented into these capillaries and completely surrounded by them. In the nephrons of the mammalian type the proximal tubule was found to be convoluted as is usual for mammalian species. The morphology of the avian kidney has been studied with casts, light microscopy, and electron microscopy (Edwards, 1940; Feldotto, 1929; Hodges, 1974; Johnson et al., 1972; Johnson and Mugaas, 1970; Kurihara and Yasuda, 1975; Ogawa and Sokabe, 1971; PakPoy and Robertson, 1957; Siller, 1981; Siller and Hindle, 1969; Sokabe et al., 1969; Sokabe and Ogawa, 1974; Spanner, 1925; Sperber, 1948, 1960),but so far only a few studies have been made on avian kidney structures from the domestic fowl fixed by perfusion (Kjaerheim, 1969; Wideman et al., 1981). In the literature there is general agreement on the kidney architecture in birds. In the avian kidney there are two different types of nephrons: a reptilian type without Henle’s loop and medullary tissue, and a mammalian type with both Henle’s loop and a renal medulla (Huber, 1917;Johnson et al., 1972; Johnson and Mugaas, 1970; Siller, 1971; Sperber, 1960). The reptilian-type nephrons are found in all areas of the kidney; in particular there is a very characteristic arrangement of the structures in the subcapsular cortex where the glomeruli lie in a semicircular array around the intralobular vein. In the deeper cortex, the reptilian-type glomeruli lie scattered between tubules and mammalian-type glomeruli (Dantzler and Braun, 1980; shoemaker, 1972; Siller, 1981; Sperber, 1948, 1960). The latter are only found in the deeper parts of the cortex. There are fewer of them than of the reptilian type and they are always located in the vicinity of the medullary cones (Johnson and Mugaas, 1970; Sperber, 1960). 0 1985 ALAN R. LISS, INC. The vascular supply of the avian kidney is double; an arterial supply from the renal arteries that terminates in the afferent arterioles of all glomeruli, and a venous supply terminating in the peritubular capillaries to the entire reptilian-type nephrons and at least the proximal tubules of mammalian-type nephrons (Kurihara and Yasuda, 1975; Siller and Hindle, 1969; Sokabe et al., 1969; Spanner, 1925; Sperber, 1948). The venous blood originates from the portal venous system (Akester, 1967; Wideman et al., 1981). The relationships between the portal venous system and the nephrons have recently been studied by injection of ferrocyanide and gelatin in the veins of the back limb (Wideman et al., 1981). However, the finer morphology of the reptilian-type nephrons is not fully understood despite one recent study with reconstruction of two reptilian-type nephrons (Wideman et al., 1981).Neither are there any reliable measurements of the kidney structures in the avian kidney. As the quality of the histological sections has been a problem in the past in connection with obtaining accurate information and measurements, the present study was performed on serial sections after whole-kidney fixation by perfusion. MATERIALS AND METHODS The kidneys from 6 domestic fowl (white Leghorns, Gallus gallus u. domesticus) were used for this study. Received August 21, 1984; accepted November 28, 1984. I. MORILD, A. BOHLE, AND J.A. CHRISTENSEN 34 The birds had been fed on a standard diet (Vestlandske Felleskjbp, Bergen, Norway), which contained 2 gm of sodium per kilogram of diet, and had free access to tapwater. They were 1.5-2 years old and weighed 1,2001,500 gm. Fixation by Perfusion On the day of sacrifice, the birds were anesthetized with sodium phenemal, 100-200 m g k g (NAF Laboratories, Oslo, Norway), injected i.v. in the alar vein. Tracheotomy was performed, and the birds were ventilated artificially. This was necessary as thoracotomy was needed to gain access to the heart, from which the kidneys were perfused (Kjaerheim, 1969). The rinsing fluid was 1.0 M phosphate buffer with 1% formaldehyde (pH 7.0). This fluid was infused for 2-3 min, and then fixation was continued with 2% glutaraldehyde and 2% formaldehyde in 0.1 M phosphate buffer (pH 7.0) for 5-6 min. The bottles with rinsing fluid and fixative were connected to each other and to a metal cylinder of compressed air. The perfusion pressure was monitored from the left ventricle with a medical transducer (SE 4-88) and amplifier (SE 4919; SE Labs EM1 Ltd., North Feltham Trading Estate, Feltham, Middlesex, England) connected to a calibrated oscilloscope (Hewlett Packard 78303A). The perfusion pressure ranged between 80 and 110 mm Hg (the mean arterial pressure in the fowl being 115 mm Hg). Histological Procedure After perfusion, kidneys were removed, cut into slices 1-2 mm thick, and placed in additional fixative for 2-3 days. Thin slices from these blocks were embedded in paraffin, sectioned serially (100-150 consecutive sections, 7-8 pm thick), and stained with periodic-acid-Schiff reagent (PAS). Similarly, a n equal number of consecutive sections from small tissue blocks embedded in plexiglass were cut (1-2 pm thick) on a h i c h e r t Jung Rotocut microtome and impregnated with silver by Movat’s method (1961). TOseparate reptilian-type from mammalian-type nephrons, tissue for semithin sections was taken from subcapsular and juxtamedullar regions of renal cortex, respectively. Fig. 1. Reconstructed reptilian-type nephron. Two-dimensional view from “above.” Black line is positioned centrally in the tubular lumen. Star indicates glomerulus. Arrow indicates beginning of the collecting duct. Reconstructions The entire tubule of eight mammalian-type nephrons was followed in serial sections from the glomerulus to the collecting duct and the course of the tubule was drawn. Four of these were selected from the subcapsular cortex and four from the deeper cortex, about halfway between the kidney surface and the renal medulla. For this purpose two schematic drawings were made of each tubule in which the tubules were seen from two angles 90” apart (from “above” and “laterally”). On the horizontal axis the distance from the interlobular to the intralobular vein was plotted on the sheets and hence the position of glomeruli, tubuli, collecting ducts and arteries were drawn. On the vertical axis the number of the sections were listed. All measurements were to scale. In this manner two-dimensional drawings like Figure 1 and Figure 2 were made. By combining the two views in Figure 1 and Figure 2, three-dimensional drawings were made (Fig. 3). Measurements Measurements on the sections were made with a morphometric program from MTS (Medizinisch Technische Apparate, D-7400 Tiibingen, FRG). This was used in connection with a computer (Commodore Computer CMB 3032, Commodore Business Machines, Santa Clara, California) and a digitizing plate (Bit Pad One TM, Summagraphics Corporation, Fairfield, Connecticut). The measurements were performed on the digitizing plate with a cursor that was equipped with a light diode. The light point from the diode was projected into the section in the microscope (Leitz Dialux 20 EB) with a tracing device. Thickness The thickness of the PAS-stained sections was estimated from the spherical glomeruli. The diameters were measured in all sections where the glomerulus was seen, 35 STRUCTURE OF THE AVIAN KIDNEY Fig. 2. Same nephron as in Figure 1 seen from a “lateral” view and the maximal diameter was divided by the number of sections. These measurements were carried out on 6 glomeruli each in 2 different series. The average thickness of the sections was 6.98 pm in one series and 7.02 pm in the other. The individual results are listed in Table 1. The thicknesss of the semithin sections was determined with a Zeiss instrument for interference microscopy using the method of Jamine and Lebedeff with Senarmont compensation (Hale, 1958)(Table 2). Measurements of the diameter of proximal and distal tubules were carried out on PAS-stained series. Fifty cross-cut proximal and 50 cross-cut distal tubules were measured. The sections were deliberately chosen from all 6 animals. Finally, the distance between the interlobular and intralobular veins was measured in the areas with reptilian-type nephrons. As these nephrons constantly showed the same pattern of primary foldings, the distance between the two veins could be used to calculate the approximate minimal length of the nephron. For this purpose we measured the distance between the inter- and intralobular vein in 180 nephrons from all 6 animals. In addition, the extent of the nephrons was estimated to obtain an impression of the area covered by a single nephron. Fig. 3. Model of the reptilian-type nephron shown in Figures 1 and 2. Note folds parallel to kidney surface at the top. Star indicates position of glomerulus. Arrowhead indicates transition from the proximal to the distal tubule. Arrow indicates beginning of the collecting duct. The mammalian-type nephrons were followed from the glomerulus through the promimal labyrinth and into, but not through, the medulla, as these nephrons had been found to be similar to the nephrons in mammalian species (Dantzler and Braun, 1980). RESULTS The avian kidney is incompletely divided into three major parts (the cranial, middle, and caudal). The structure of the kidney tissue, which is the same in all three parts, is lobular, and each lobule is outlined by the ramifications of the interlobular veins of the renal portal system. This lobular subdivision of the kidney was found in the subcapsular and middle areas of the cortex. Here all nephrons were of the reptilian type arranged in a horse- I. MORILD, A. BOHLE, AND J.A. CHRISTENSEN 36 TABLE 1. Average thickness of PAS-stained sections estimated from the diameters of reptilian-type glomeruli No. of Avg. thickness glomeruli of sections (fim) X+SD Series No. H.3 28.01.82 H.2 04.02.82 6 6.3,g.l 6.5, 5.6 6.7, 7.7 6.1,6.9 7.4, 6.6 7.5. 7.6 6 6.98 k 1.24 7.02 0.59 TABLE 2. Thickness of semithin sections measured by interference microscopy Measured values Sections cut at 1pm Sections cut at 2 pm n = n in fim 10 1.0, 1.0,1.0,1.0, 1.0, 1.2, 1.0, 1.1,1.0, 1.1 2.0, 2.3,2.3,2.1, 2.1, 2.2, 2.2 2.2,2.1,2.2 10 X SD - 1.04 0.07 2.17 f 0.09 Number of sections measured. shoe pattern around the intralobular vein (Fig. 4). The reptilian-type glomeruli were found about halfway between the interlobular and intralobular veins. Originating from the glomerulus, the proximal tubule first ran peripherally in the lobule to the interlobular vein. Here it turned and ran to the center of the lobule, turned again, and ran all the way back to the interlobular vein. The proximal tubule of the reptilian-type nephrons thus had no convoluted part (Fig. 5). However, this pattern could be modified to a very great extent. In the subcapsular nephrons the proximal tubule in particular often ran parallel to the kidney surface for more than 300 pm (Fig. 3). Despite this variation, the major bends were always present. After having turned once more, the tubular lumen became wider. The epithelial lining was then flatter and the cells had a lighter staining cytoplasm. This was the junction between the proximal and distal tubule, and the change occurred often, but not always, very abruptly from one cell to the next. No loop of Henle could be identified, but the distal tubule always ran to the glomerular hilus, where it had direct contact with the vascular pole. On the side of the distal tubule where it was attached to the vascular pole, the epithelial cells had a different appearance from that seen elsewhere in the distal tubule. The cells were somewhat taller and more narrow, with closely packed nuclei. The cytoplasm of these cells mostly stained lighter than that of the neighboring cells. Thus these cells showed the characteristics of macula densa cells (Fig. 6). Postglomerularly the distal tubule often became very thin-walled and was almost always convoluted. However, as in the proximal tubule large variations were seen. These ranged from a U-shaped loop to a labyrinth of folds. This part of the nephron always ran close to the intralobular vein with large venous branches that came from the interlobular vein between the segments of the distal tubule (Fig. 4). As the distal tubule of the deeper glomeruli turned the last time, it was almost within the Fig. 4. Cortical lobule with reptilian-type glomeruli arranged in a horseshoe pattern around the intralobular vein (i). Note large venous channels between distal tubules in the center of the lobule. Micrograph X 70, silver methenamine. intralobular vein. From this point it ultimately ran towards the collecting duct, which it reached in the outer third of the lobule. On the way between the central vein and the collecting duct, the distal tubule exhibited a thin segment with a n extremely flat epithelium reminiscent of the thin loop of Henle (Fig. 7). After some distance (50-150 pm) the epithelium was higher again and finally the distal tubule drained into the collecting duct. This composition of the reptilian-type nephron resulted in a long structure lying between the two great veins (Figs. 2,3).We found the outer and inner diameter of the tubule to change frequently within the same tubular segment, between different tubular segments, and from tubule to tubule. The venous drainage from the interlobular to the intralobular vein occurred via a venous capillary network between the tubular segments. In the periphery of the lobule the tubules lay close together and the veins were narrow. More centrally the tubules were separated by large capillaries with increasing lumina that eventually resulted in large venous channels towards the intralobular vein. The distal convoluted tubule was always surrounded by these venous channels. The termination of STRUCTURE OF THE AVIAN KIDNEY 37 these peritubular venous channels in the intralobular vein from many different directions created a picture in which segments of the distal tubule were found indented into the intralobular veins (Fig. 8). The average area covered by one reptilian-type nephron was about 880 pm long and 115 km wide (Table 3). The diameter of the proximal tubule was 40.8 pm, that of the distal tubule 23.1 pm, and that of the collecting duct 38.2 pm. The total length of the nephron was difficult to estimate because of the convoluted parts and because the sections will always be at an angle to the nephron, but it was found to be at least 2,400 pm long (Table 3). The length of the tubule was also measured by means of the reconstructions. Here it was found to be slightly more than 6,000 pm in the longest and most complicated folded nephron (Fig. 3) and approximately 3,000 pm in the shortest nephrons with few secondary folds. In the mammalian-type nephrons the proximal tubule was convoluted, as is usual in mammalian species. The distal tubule showed a definite macula densa with taller and more narrow cells on the glomerular side of the lumen. Other typical hallmarks of the macula densa such as crowding of their nuclei and lighter-staining cytoplasm were also present (Fig. 9). DISCUSSION loop W Fig. 5. Schematic drawing of the basic folding pattern of the reptilian-type nephron in the avian kidney. For better orientation the tubular loops are drawn side by side. Glomerulus and proximal tubule dotted. Distal tubule white. Arrowheads indicate interlobular and intralobular veins. The work of Huber (1917) on corrosion casts revealed many important features of the avian nephrons. However, the fine relationships of the tubular segments could not be disclosed because the technique Huber had used forced him to tear the nephrons apart from each other. The work of Wideman et al. (1981)was very comprehensive in this respect and we were able to confirm the basic folding pattern of the reptilian-type nephrons described by these authors (Fig. 5). The conclusions in their study, however, were drawn mainly on the reconstruction of two subcapsular nephrons. The present study Fig. 6. Reptilian-type glomerulus with juxtaglomerular apparatus. Macula densa in the distal tubule between arrowheads. Between the distal tubule and the glomerulus the Goormaghtigh cells are seen (Christensenet al., 1982). Micrograph ~ 4 5 0silver , methenamine. 38 I. MORILD, A. BOHLE, AND J.A. CHRISTENSEN Fig. 7. Reptilian-type distal tubule with transition of epithelium from cuboid to flat type (arrowheads).Micrograph ~ 4 5 0PAS. , Fig. 8. Intralobular vein with distal tubular segments in large venous channels splitting up the boundary of the vein. Arrow points to an endothelial nucleus. Micrograph ~ 2 8 0silver , methenamine. analyzed the nephrons more precisely, as we reconstructed 8 nephrons from different birds and in different regions of the renal cortex. The basic architecture was maintained in all the reconstructed nephrons, but the individual variations were very large. No single explanation was found for these variations, but the cortical location may be a n important factor because the largest loops were seen in the subcapsular nephrons. It seemed, however, more likely that the secondary folds, i.e. those additional to the basic pattern, were determined by many factors such as neighboring nephrons and the distance between the glomeruli and the renal surface. STRUCTURE OF THE AVIAN KIDNEY 39 Fig. 9. Mammalian-type glomerulus with distal tubule and juxtaglomerular apparatus on the top. Macula densa indicated with arrowheads. Between the distal tubule and the glomerulus are the Goorrnaghtigh cells. Micrograph ~ 2 8 0silver , methenamine. TABLE 3. Measurements of reptilian-type nephron outlines and tubular diameters Nephron outline length Nephron outline breadth Diameter prox. tubule Diameter dist. tubule Diameter coll. duct n = n Observations per animal 6 30 6 30 6 50 6 50 6 50 Mean per animal 890,946, 727, 921,959,875 105,108,116 102,125,126 43.6,40.7,37.6, 41,7,45.7,35.4 27.9,21.8, 19.1, 25.5, 24.5, 19.7 45.5, 35.6, 33.7, 36.2,44.8,33.6 Mean of group SD 886 & 84.4 114 k 10.3 40.8 + 3.8 23.1 f 3.5 38.2 & 5.5 Number of animals. All figures in microns. The distance between the glomeruli and the intralobular veins may also be of significance to the secondary tubular foldings. The size of the glomeruli is another parameter that may influence the glomerular filtration, and secondary tubular length and foldings. This means that a certain tubular length is of functional importance with respect to reabsorption and excretion and will always be established even if the tubules have to make the most peculiar bends, loops, and foldings. Particularly in the subcapsular region of the cortex, the proximal tubule was folded parallel to the kidney surface (Fig. 3). In our reconstructions we found much greater variation of the tubular architecture than did Wideman et al. (1981).We, too, found an abrupt transition between the proximal and distal tubule but not in all nephrons. The measurements of tubular diameter showed that the proximal tubule and the collecting duct were approximately the same size. The distal tubule had a diameter half that of the proximal tubule. We also found conspicuous variations of the outer tubular diameter on all levels of the nephrons. These changes were combined with variations in epithelial differentiation. Thus, narrow tubules had a more flattened epithelium than wide tubular segments. Very often, but not always, the distal tubule of the reptilian-type nephrons in all regions of the cortex had a very thin segment (Fig. 7). Here the epithelium was so flat that the nuclei elevated the luminal surface of the cells. This segment was found shortly after the distal tubule had made its last bend close to the intralobular vein and was running towards the collecting duct. This segment was reminiscent of the 40 I. MORILD, A. BOHLE, AND J.A. CHRISTENSEN thin loop of Henle, but as Henle’s loop is located between the proximal tubule and the distal part of the tubule in mammalian-type nephrons, anatomically the thin segment of the reptilian-type nephron in the bird belonged to another part of the tubule. The length of the tubule did not correspond to the results of Huber (1917), as his figures are much higher than ours. Wideman et al. (1981) calculated the length of the nephrons from their reconstructions. Their nephron lengths were 2,016 pm and 2,375 pm. However, when the given scale in their publication is compared with the illustrations of the reconstructed nephrons, it seems as if the nephron lengths were approximately 20% shorter. These discrepancies may be caused by the fact that we measured on the reconstructions, whereas Wideman et al. (1981) may have measured on serial sections, although this is not stated in the text. When the distal tubule reached the glomerular hilus, a macula densa was always present. This was true for both the reptilian-type and mammalian-type nephrons. This finding is in accordance with most of the others reported (Berger, 1966;Edwards, 1940;Johnson and Mugaas, 1970; McKelvey, 1963; Schoemaker, 1972; Siller, 1971).However, in these studies the reptilian-type nephrons were not distinguished from the mammalian-type. A recent publication (Wideman et al., 1981) on renal tissue fixed by perfusion failed to demonstrate a macula densa. This may have been caused by the fact that their study was made on semithin sections alone. In our opinion it is difficult to recognize the macula densa on semithin sections without the experience of serial sections from material embedded in paraffin. Ogawa and Sokabe (1971) found the macula densa cells in birds to be intermediate in structure between macula densa cells and ordinary tubular cells. They did not distinguish between reptilian- and mammalian-type nephrons and used fixation by immersion. Unpublished data from our laboratory indicate that the subcellular organization of the distribution of microchondria and folds of the basal labyrinth is similar in both macula densa cells and in other distal tubular cells. There are only narrower and taller cells in the macula densa segment. ACKNOWLEDGMENTS This study was supported by the Gade Foundation and the German Research Foundation. I. Sdborg prepared all the serial sections with great care and L. WieseHansen typed the manuscript. LITERATURE CITED Akester, A.R. (1967) Renal portal shunts in the kidney of the domestic fowl. J. Anat., lOlr569-594. Berger, C. (1966)Mikroskopische und histochemische Untersuchungen an der Niere von Columbia Livia aberratio domestica. 2. mikrosk. Anat., 54:436-456. Christensen, J.A., I. Morild, E. Mikeler, and A. Bohle (1982) Juxtaglomerular apparatus in the domestic fowl (Gallus domesticus). Kidney Int., 22(Suppl. 12):S24-S29. Dantzler, W.A., and E.J. Braun (1980) Comparative nephron function in reptiles, birds and mammals. Am. J. Physiol., 8(2):R197-R213. Edwards, J.G. (1940) The vascular pole of the glomerulus in the kidney of vertebrates. Anat. Rec., 76:381-389. Feldotto, A. (1929) Die Harnkanalchen des Hunes. 2. Mikrosk. Anat. Forsch., 17:353-370. Hale, A.J. (1958) The Interference Microscope in Biological Research. E. and S. Livingstone, Edinburgh, London. Hodges, R.D. (1974) The histology of the fowl. In: The Urinary System. Academic Press, London, New York, San Francisco, pp 489-524. Huber, G.C. (1917) On the morphology of the renal tubules of vertebrates. Anat. Rec., 13r305-339. Johnson, O.W. (1979) Form and Function in Birds. In: Urinary Organs. A.S. King and J. McLelland, eds. Academic Press, New York, London, Vol. 1, pp 183-235. Johnson, O.W., and J.N. Mugaas, (1970) Some histological features of avian kidneys. Am. J. Anat., 127r423-436. Johnson, O.W., G.L. Philips, and J.N. Mugaas (1972) Injection studies of cortical and medullary organization in the avian kidney. J. Morphol., 136:181-190. King, A.S. (1975)Aves urogenital system. The anatomy of the domestic animals. In: Sisson and Grossman’s: The Aves. 5th Ed. R. Getty, ed. W.B. Saunders Philadelphia, London, Toronto. Kjaerheim, A . (1969) Studies of adrenocortical ultrastructure. 1. Aldehyde perfusion fixation of the domestic fowl. Acta Anat., 74t424453. Kurihara, S., and M. Yasuda (1975) Morphological study of the kidney in the fowl. I: Arterial system. Jpn. J. Vet. Sci., 3729-47. McKelvey, R.W. (1963) The presence of a juxtaglomerular apparatus in non mammalian vertebrates. Anat. Rec., 145259-260. Movat, H.Z. (1961) Silver impregnation methods for electron microscopy. Am. J. Clin. Pathol., 35228-257. Ogawa, M. and H. Sokabe (1971) The macula densa site of avian kidney. Z. Zellforsch., I2Or29-36. PakPoy, R.K.F., and J.D. Robertson (1957) Electron microscopy of the avian renal glomerulus. J. Biophys. Biochem. Cytol., 3:183-203. Gchoemaker, V.H. (1972) Osmoregulation and excretion in birds. In: Avian Biology. D.S. Farner and J.R. King, eds. Academic press, New York, London. Vol. 2, pp. 527-574. Siller, W.G. (1971) Structure of the kidney. In: Physiology and Biochemistry of the Domestic Fowl. D.J. Bell and B.M. Freeman, eds. Academic Press, New York, London, Vol. 1.pp. 197-231. Siller, W.G. (1981) Renal pathology of the fowl-A review. Avian pathol. 10r187-262. Siller, W.G., and R.M. Hindle (19691 The arterial blood supply to the kidney of the fowl. J. Anat., 104t117-135. Sokabe, H., M. Ogawa (1974) Comparative studies of the juxtaglomerular apparatus. Int. Rev. Cytol., 37:271-327. Sokabe, H., M. Ogawa, M. Oguri, and H. Nishimura, (1969) Evolution of the juxtaglomerular apparatus in the vertebrate kidneys. Texas Rep. Biol. Med., 27r857-885. Spanner, R. (1925) Der Pfortaderkreislauf in der Vogelniere. Morphol. Jahrb., 54t560-632. Sperber, J. (1948)Investigations on the circulatory system in the avian kidney. Zool. Bidrag Uppsala, 27:429-448. Sperber, J. (1960) Excretion. In: Biology and Comparative Physiology of Birds. A.J. Marshall, ed. Academic Press, New York, London, Vol. 1, pp. 469-492. Sutherland, L.E. (1966) Immunological and Functional Aspects of Juxtaglomerular Cells. Doctoral thesis, University of Toronto. Taylor, A.A., J.O. Davis, R.P. Breitenbach, and P.M. Hartroft, (1970) Adrenal steroid secretion and a renal-pressor system in the chicken (Gallus domesticus). Gen. Comp. Endocrinol, 14r321-333. Wideman, R.F., E.J. Braun, and G.L. Anderson, (1981) Microanatomy of the renal cortex in the domestic fowl. J. Morphol., 168249-267.