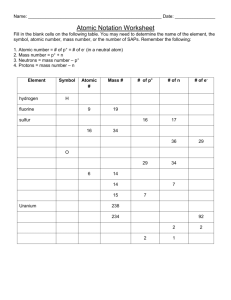
1. The 3 particles of the atom are
advertisement

1. The 3 particles of the atom are: Their respective charges are: a. ___proton__________ a. __positive (+) _______ b. ___neutron___________ b. __neutral_(0)________ c. ___electron____________ c. __negative_(-)_______ 2. The number of protons in one atom of an element determines the atom’s __atomic number________ , and the number of electrons determines _ net charge_________ of an element. 3. The atomic number tells you the number of _____protons_________ in one atom of an element. It also tells you the number of ____electrons________ in a neutral atom of that element. The atomic number gives the “identity “of an element as well as its location on the Periodic Table. No two different elements will have the _____same_____________ atomic number. 5. The _______mass number_____ of an element is the total number of protons and neutrons in the_ nucleus_______________ of the atom. 6. The mass number is used to calculate the number of ___neutrons__________ in one atom of an element. In order to calculate the number of neutrons you must subtract the __atomic number___________ from the ______mass number_____________ . 7. Give the symbol and number of protons in one atom of: Lithium Iron 3Li 92U Bromine 35Br Copper 29Cu 80Hg Oxygen 8O Mercury Krypton 36Kr Helium 2He 8. Give the symbol and number of electrons in a neutral atom of: Uranium U 92 electrons Boron B 5 electrons Antimony Sb 51 electrons Chlorine Cl 17 electrons Iodine I 53 electrons Xenon Xe 54 electrons 9. Give the symbol and number of neutrons in one atom of: (To get “mass number”, you must round the “atomic mass” to the nearest whole number) Barium Ba 81 neutrons Bismuth Bi 126 neutrons Carbon C 6 neutrons Hydrogen H 0 neutrons Fluorine F 10 neutrons Magnesium Mg 12 neutrons Europium Eu 89 neutrons Mercury Hg 121 netrons 10. Name the element which has the following numbers of particles: a. 26 electrons, 29 neutrons, 26 protons Iron (Fe) b. 53 protons, 74 neutrons Iodine (I) c. 2 electrons (neutral atoms) Helium (He) Remember when using the Periodic Table--To get “mass number”, you must round the “atomic mass” to the nearest whole number Number Number Number Element Atomic Mass Symbol of of of name number number Protons Neutrons Electrons Helium H O N3C 1+ Kr 2+ U Ne In Cr Nitrogen Carbon Na Cu Oxygen 4+ Ca Sr Pd or Ag V Sodium Krypton Copper Uranium Neon Indium Chromium Calcium Strontium Palladium Silver Vanadium 2 4 2 2 2 8 16 8 8 8 7 14 7 7 10 6 14 6 8 8 11 23 11 12 10 36 81 36 45 36 29 64 29 35 27 92 238 92 146 92 20 10 10 10 49 115 49 66 49 24 52 24 28 24 41 20 21 20 38 88 38 50 38 46/47 107 46/47 61/60 46/47 23 51 26 25 23 10 20