human immunoglobulin glycosylation and the lectin pathway of
advertisement

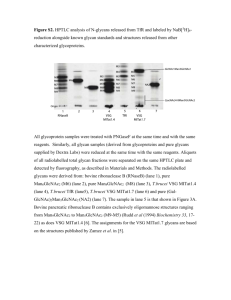
9 HUMAN IMMUNOGLOBULIN GLYCOSYLATION AND THE LECTIN PATHWAY OF COMPLEMENT ACTIVATION James N. Arnold1, Louise Royle2, Raymond A. Dwek2, Pauline M. Rudd2, and Robert B. Sim1 1MRC Immunochemistry Unit 2Oxford Glycobiology Institute Department of Biochemistry University of Oxford South Parks Road, Oxford OX1 3QU, UK 1. INTRODUCTION Immunoglobulins are the major secretory products of the adaptive immune system. They are glycoproteins which are found in all higher vertebrates (mammals, birds, reptiles, amphibians, bony and cartilaginous fish, but not in jawless fish (agnatha)) (Litman et al., 1999). In humans there are five classes IgG, IgM, IgA, IgE and IgD. The immunoglobulins share similar structures (Fig. 1). Each immunoglobulin molecule is composed of two identical disulphide bridged class-specific heavy chains, each disulphide bridged to a light chain of which there are two isoforms named k and l. Both heavy and light chains are composed of regions called immunoglobulin domains. The immunoglobulin fold/domain is about 105–120 amino acids long and is composed of b-sheet secondary structure (Amzel and Poljak, 1979). The role of immunoglobulins is to bind to antigens via their N-terminal (variable amino acid sequence) domains and to mediate effector ff functions, such as activation of complement (Malhotra et al., 1995; Roos et al., 2001) or binding to receptors via their constant (invariable sequence) domains (Mimura et al., 2000; Shields et al., 2001). During immunoglobulin synthesis, rearrangement of gene segments and somatic mutation creates variation in amino acid sequence in the N-terminal domains (named VH and VL domains for Variable Heavy and Light chains respectively). The light chains have one V domain and one constant sequence domain (CL). The sequence of all l chain C domains is the same, and the sequence is homologous to 27 John S. Axford (ed.), Glycobiology and Medicine, 27-43. © 2005 Springer. Printed in the Netherlands. 28 J. N. Arnold et al. Figure 1. Immunoglobulin Structure a) The structure of IgG showing the Variable Heavy (VH) and Constant Heavy (CH), Variable Light (VL) and Constant Light (CL) domains. The diagram identifies the Fab, Fc and flexible hinge regions of the molecule. This hinge varies in length between the different ff immunoglobulin classes and is replaced by additional CH domain in IgE and IgM. The approximate positioning of the Asn-297 N-linkage site for glycans is marked. b) Diagrammatic representation of IgG1, IgD, IgA1, IgE and IgM showing N- and Olinked glycan positions, and inter-chain disulphide bridges. The domains themselves contain intra-domain disulphide bridges, although these are not marked. IgM circulates in the serum in both pentameric and hexameric forms, in which the monomeric units are disulphide bridged together. Pentameric IgM contains a single J chain but hexameric IgM does not (Weirsma et al., 1998). Human Immunoglobulin Glycosylation 29 the C domain shared by all k chains. Heavy chains have 3 or 4 C domains. The sequences of the C domains are class or subclass specific, i.e. all IgGs have identical constant regions, as do all IgMs. Each clone of B lymphocytes secretes only one immunoglobulin molecule, which has V regions unique to that particular B cell clone. Total IgG isolated from human serum therefore contains 4 subclasses, each with similar but distinct constant regions, and with 105–106 different ff V region sequences. IgM and IgD occur both as soluble forms (in serum) and membrane-bound forms on B lymphocytes (Van Boxel et al., 1972). The membrane-bound forms have an additional trans-membrane segment, C-terminal to the constant regions. IgA, IgG, IgE are all soluble molecules: IgG is the most abundant in serum (10–15 mg/ml), while IgA is the most abundant immunoglobulin overall. Most IgA is secreted through epithelia into the mucous lining of the gastrointestinal and respiratory tract, and into tears, saliva and milk (Norderhaug et al., 1999). The secreted form is generally dimeric and contains an extra glycosylated polypeptide chain, SC (Secretory Component) and glycosylated 16KDa J chain (Johansen et al., 2001; Royle et al., 2003), which is also found in pentameric forms of IgM (Wiersma et al., 1998). The single J chain is disulphide bridged to two C-termini of both IgM and IgA molecules (Wiersma et al., 1998; Royle et al., 2003). IgA in serum is predominantly monomeric but also forms dimers and higher polymers (Delacroix et al., 1982; Roos et al., 2001). IgE is the lowest abundance immunoglobulin, occurring as a monomer at <1 mg/ml. IgD also occurs as a low abundance monomer in serum at <30 mg/ml, while IgM is at high concentrations (~2.5 mg/ml). IgM occurs predominantly as pentamers and hexamers, although a small amount of monomer also circulates (Sørensen et al., 1999). ff classes of immunoglobulin are distinct in their major effector ff The different functions. IgM is principally associated with complement classical pathway activation via binding of C1q (Wiersma et al., 1998). IgG also activates complement via classical (Duncan and Winter, 1988) and alternative pathways (Anton et al., 1989) and mediates ADCC (Antibody Dependent Cell Cytotoxicity) (Sarmay et al., 1992). IgE is associated with mast cell and basophil stimulation in allergic conditions. IgA in secretions may act mainly to agglutinate (immobilise) or neutralise micro-organisms (Lamm, 1997). No effector ff functions have been identified for IgD. In addition to their enormous diversity of amino acid sequences and antigenbinding specificity, immunoglobulins display considerable diversity in the location and number of glycosylation sites (both N- and O-linked) and great diversity in glycan structure. The glycans attached to the immunoglobulins are important for immunoglobulin solubility (Tarentino et al., 1974), subcellular transport and secretion (Gala and Morrison, 2002), conformation (Mimura et al., 2000), binding to Fc receptors (Mimura et al., 2000), normal plasma clearance (Skockert, 1995) and complement activation (Malhotra et al., 1995). This chapter discusses both the glycan structures that are attached to the normal human serum immunoglobulins and their potential roles in complement activation through the binding of the serum ‘recognition’ lectin, Mannan Binding Lectin (MBL), and the subsequent activation of the lectin pathway of the complement system. 30 J. N. Arnold et al. 2. GLYCOSYLATION OF THE IMMUNOGLOBULINS 2.1. IgG There are four subclasses of IgG, named IgG1–4, that differ ff in their heavy chain constant region sequence and disulphide bridging. The subclasses have distinctive glycan pools (Jefferis ff et al., 1990). All IgGs have a single N-linked glycosylation site on each heavy chain in the CH2 domain at Asn-297 (Fig. 1). There are no conserved glycosylation sites in the light chain or variable regions of the heavy chain. The glycan population attached at Asn-297 contains three sets of glycoforms termed IgG-G0, -G1 and -G2 (Fig. 2b). The IgG-G2 biantennary glycans occupying Asn-297 have two arms that both terminate in galactose residues. This set of glycoforms accounts for approximately 16% of total IgG glycans. Approximately 35% are IgG-G1, which lack a terminal galactose residue on one biantennary arm, exposing a GlcNAc residue. IgG-G0 glycans make up 35% and neither biantennary arm contains a galactose residue. The final 14% of serum IgG glycans consist of IgG-G2 or -G1 glycoforms which are sialylated. Within the glycans of IgG there is a diversity of structures caused by the presence of bisecting GlcNAc residues (B in Fig. 2a) (approximately 30% of total IgG1 glycan pool), core fucose (Fc in Fig. 2a) (approximately 70% of the total IgG1 glycan pool) and sialylation of the terminal 1,3 arm galactose residues (S in Fig. 2a) (14% of total IgG1 glycan pool) (Butler et al., 2003)). IgG1 is the most abundant subclass of IgG in the serum. IgG2 and IgG3 have a preferred linkage of the galactose residues to the a1,3 arm mannose, whereas IgG1 has preferential linkage of galactose to the a1,6 arm mannose. IgG4 is reported to contain predominantly fully galactosylated structures (Jefferis ff et al., 1990). There is considerable amino acid sequence diversity in the variable regions, and N-linked glycosylation sites can occur in the variable regions. These are relatively rare. A recent survey of heavy chain variable region cDNA sequences showed that only 7 out of 75 (9.3%) had a potential N-linked glycosylation site in the variable region (Zhu et al., 2002). The glycans that occupy these sites are predominantly sialylated structures, with a high incidence of bisecting GlcNAc residues (Youings et al., 1996.; Wormald et al., 1997). 2.2. IgM IgM is found predominantly in the serum as a pentameric structure disulphide bridged at the CH3 domains and at the tail piece (a flexible region following the CH4 domain) and believed to form a ring structure. Pentameric IgM also has a J chain that contains a single N-linked glycosylation site. IgM can also adopt a hexameric structure that contains no J chain (Wiersma et al., 1998). IgM heavy chain (m chain) has five N-linked glycosylation sites at Asn-171, Asn-332, Asn-395, Asn-402, and Asn-563. Asn-402 and Asn-563 have been shown to be occupied by oligomannose structures (Chapman and Kornfeld, 1979; Wormald et al., 1991). The other N-linked glycosylation sites on each m chain in normal human serum IgM are occupied predominantly by complex biantennary glycans. The most predominant glycan is FcGlcNAc A G S (26% of total glycan pool). Sialylated structures 2 2 2 1 Human Immunoglobulin Glycosylation 31 Figure 2. Glycan Structure and IgG Glycoforms. a) Shows the general nomenclature used to describe sugar residues, bond angles and sugar linkages of the ff glycan structures that occupy glycoproteins. b) Shows the predominant glycan structures that different occupy the Asn-297 site in IgG. The glycans shown may also vary by the presence of absence of a core Fucose and/or bisecting GlcNAc. 32 J. N. Arnold et al. Figure 3. IgA Glycosylation Types. IgA has two subclasses, IgA1 and IgA2, and both have N-linked glycosylation at Asn-263 and Asn-459. IgA1 contains nine potential O-linked sites in the hinge region, of which five or six have been shown to be occupied. *The sixth O-linked site occupies one or more of Ser-224, Thr-233, Ser-238 or Ser-240 (Tarelli et al., 2004). IgA2 has no potential O-linked sites in its hinge region. IgA2 is subdivided into IgA2m(1) which has two additional N-linked glycosylation sites in the CH1 domain and CH2 domain, and IgA2m(2) which has these additional N-linked sites but also a third additional CH1 domain N-linked glycosylation site. (61.8%), core fucosylated (65%) and bisected structures (38%) are present in the total glycan pool (J.Arnold unpublished data). 2.3. IgA IgA has two conserved N-linked glycosylation sites, at Asn-263 in the CH2 domain and Asn-459 located in the 18 amino acid tail piece on each a chain. There are two subclasses of IgA designated IgA1 and IgA2. IgA2 has two forms that contain two (IgA2m(1)) or three (IgA2m(2)) extra conserved N-linked glycosylation sites respectively (Fig. 3). IgA occurs in several different ff oligomeric forms, and is present both in serum and in secretions. Serum IgA and Secretory IgA (SIgA), have distinct populations of glycan structures. The 23 amino acid hinge region in IgA1 contains nine potential O-linked sites of which five have been shown to be occupied (Mattu et al., 1998: Baenziger and Kornfeld, 1974b). These sites are Thr-228, Ser-230, Ser-232, with Thr-225 and Thr-236 Human Immunoglobulin Glycosylation 33 Figure 4. Core I Structures, Neutral, Mono-, and Di-Sialylated. The neutral, mono- and di-sialylated Core I O-linked glycan structures, that have been identified on serum IgA1 and also IgD hinge regions. The nomenclature is explained in Fig. 2. partially occupied (Mattu et al., 1998). Recently a sixth occupied O-linked site at one or more of Ser-224, Thr-233, Ser-238 or Ser-240 (Tarelli et al., 2004) has been identified. No O-linked glycans have been identified on IgA2. 2.3.1. Serum IgA Serum IgA consists mainly of IgA1. IgA1 and IgA2 contain similar N-linked glycan structures (Endo et al., 1994; Royle et al., 2003). Over 80% of the glycans are di-galactosylated bi-antennary complex glycans. Less than 10% are tri- and tetra-antennary structures (Mattu et al., 1998). Sixty four percent of the glycan structures are sialylated and 95% of these are linked a2–6 to galactose. The predominant glycan is GlcNAc A G S (24%). The glycan pool has 36% of the glycans 2 2 2 2 containing a core fucose residue and 25% containing a bisecting GlcNAc residue. The oligosaccharides attached to the Fab in IgA2 differ ff from those that occupy the Fc in for example, the presence of triantennary structures and outer-arm fucose residues such as GlcNAc A G FS which accounts for 3.7% of total Fab glycan 2 3 3 3 pool (Mattu et al., 1998). The O-linked glycans on the heavy chain of IgA1 have been identified (Mattu et al., 1998, Field et al., 1994 and Rudd et al., 1994)). The hinge is predominantly occupied by mono-sialylated core I structures (37%) and neutral core I structures (31%) (Mattu et al., 1998) (Fig. 4). 2.3.2. Secretory IgA SIgA is a dimer, held together with a J chain (which has one N-linked site) and Secretory Component (SC). The SC is the extracellular portion of the epithelial polymeric Ig receptor (pIgR), and is required for transcytosis of the IgA across the epithelium to the mucosal surface. The SC has seven N-linked glycosylation sites. SIgA contains both IgA1 and IgA2 populations. SIgA is present in mucosal secretions such as colostrum and milk and can bind to microorganisms, their metabolic products and toxins, preventing their attachment 34 J. N. Arnold et al. to the epithelium and facilitating their excretion. This process is known as immune exclusion (reviewed by Lamm, 1997). The N-linked glycans from the heavy chain of colostrum SIgA consist of approximately 15% sialylated structures (solely a2–6 linked sialic acids), with over 75% of structures containing a bisecting GlcNAc and 50% being core fucosylated. Oligomannose structures account for 12% of the N-linked glycan pool. There is a lack of glycan processing of the N-linked glycans on the a-chain of SIgA, as only 20% of structures are fully galactosylated, and 66% have an exposed terminal GlcNAc residue. The major structures occupying SIgA heavy chain are FcGlcNAc A B (30%), GlcNAc A B (21%) and FcGlcNAc A BG (8%) (Royle 2 2 2 2 2 2 1 et al., 2003). There is a large diversity of O-linked glycan structures on the heavy chain of SIgA1, which contains over 50 different ff structures of up to 15 residues in size (Royle et al., 2003), in contrast to the restricted pool of structures present on serum IgA1. The glycans occupying the J chain single N-linked site are predominantly sialylated biantennary structures (75%). Fifty percent of all structures are core fucosylated, and 50% of the neutral structures contain a bisecting GlcNAc residue (Royle et al., 2003). Interestingly no bisecting GlcNAc is present on the sialylated structures (Royle et al., 2003). The seven N-linked sites of the SC are occupied by a large diversity of structures, many of which are not found on the immunoglobulin heavy chains, for example, outer-arm fucosylated glycans. The presence of these structures may be explained partially by the fact that epithelial cell glycosylation machinery glycosylates the SC, whereas the plasma cell glycosylates the immunoglobulin. The SC N-linked glycans are predominantly bi-antennary structures. Tri-antennary (11.7%) and tetra-antennary (<1%) structures are also present (Royle et al., 2003). Over 70% of the glycans are sialylated, predominantly mono-sialylated structures, and over 65% of the glycans contain a core fucose (Royle et al., 2003). 2.4. IgD IgD has three N-linked glycosylation sites in the Fc at Asn-354, Asn-445, Asn-496 (Takahashi et al., 1982). Asn-354 in the CH2 domain is occupied solely by oligomannose structures (GlcNAc Man ) (Mellis and Baenziger, 1983a; Arnold 2 5-9 et al., 2004) which represent 34% of the total glycans. The predominant oligomannose structure is GlcNAc Man . Glucosylated mannose structures (GlcNAc Man Glc , 2 8 2 9 1 GlcNAc Man Glc and GlcNAc Man Glc ) are also present (Arnold et al., 2004; 2 8 1 2 7 1 Mellis and Baenziger, 1983a). The other 66% of glycan structures have been shown to terminate in galactose or sialic acid. These glycans occupy the two CH3 domain N-linked glycosylation sites Asn-445 and Asn-496. At these two CH3 N-linked sites 71% of the oligosaccharides are sialylated; both mono- (53%) and di-sialylated (47%) glycans have been identified. Twenty nine percent of glycans terminate in galactose residues, 50% contain core fucosylation and 50% of the glycans contain a bisecting GlcNAc (Arnold et al., 2004) at these two sites. The hinge region of IgD contains several potential O-linked glycosylation sites. In an IgD myeloma protein IgD:WAH, O-linked glycans occupy Ser-106 and Thr-126, -127, -131 and -132, although it is uncertain if Thr-131 and –132 are both occupied Human Immunoglobulin Glycosylation 35 (Mellis and Baenziger, 1983b; Takahashi et al., 1982). Another myeloma IgD:NIG-65 contains seven O-linked glycosylation sites; the five identified in IgD:WAH and also Ser-110 and Thr-113 (Takayasu et al., 1982). The O-linked glycans present on the hinge region are solely Core I structures: di-, mono-sialylated and neutral structures (Fig. 4) (Arnold et al., 2004; Mellis and Baenziger, 1983b). 2.5. IgE IgE has seven N-linked glycosylation sites in the e chain at Asn-140, Asn-168, Asn-218, Asn-265, Asn-371, Asn-383, Asn-394 (Dorrington and Bennich, 1978). The Asn-394 N-linked glycosylation site is occupied solely by oligomannose structures (Dorrington and Bennich, 1978; Baenziger and Kornfeld, 1974b). The predominant oligomannose structure is GlcNAc Man (8.3% of the total glycan pool). The other 2 5 six exposed glycosylation sites on each e chain are occupied predominantly with sialylated glycan structures (46% mono- 42% di-sialylated structures), 12% galactose terminating structures, 68% core fucosylated and 14% bisected structures (Arnold et al., 2004). 3. MANNOSE BINDING LECTIN (MBL) AND THE LECTIN PATHWAY A OF COMPLEMENT ACTIVATION 3.1. MBL MBL (Fig. 5) is a glycoprotein, also known as Mannan/Mannose Binding Protein and is member of the collectin family of proteins (Malhotra et al., 1994). Collectins are large oligomeric proteins with multiple lectin domains and collagenous regions. MBL is synthesized in the liver and secreted into the blood stream. MBL is an important component of the innate immune system, which binds calciumdependently to sugars that have hydroxyl groups on the carbon-3 and carbon-4 orientated in the equatorial plane of the pyranose ring (Weis et al., 1992). This gives MBL affinity for mannose, fucose and N-acetyl glucosamine (GlcNAc) (Turner et al., 1996). This specificity allows MBL to bind to sugar arrays on the surfaces of microorganisms, including bacteria, viruses and fungi (Holmskov et al., 1994), but not to human glycoprotein glycans, the structures of which generally terminate in galactose or sialic acid. MBL has a structure and function similar to that of C1q, the recognition molecule that initiates the classical pathway of complement. MBL binds to sugar residues via the Carbohydrate Recognition Domain (CRD) (lectin) heads. The affinity of a single CRD for carbohydrate is very weak (10−3M) (Iobst et al., 1994). Multiple CRD binding leads to a much greater avidity. Levels of MBL in human serum vary greatly between individuals (Turner, 1996), from below 50ng/ml to above 10ug/ml. The variation of MBL levels is caused by several identified polymorphisms in the coding sequence and promoter regions of the MBL gene (Madsen et al., 1995). The coding sequence polymorphisms disrupt the Gly-X-Y repeat that is found in the collagenous region destablilising the collagen triple helix formation (Sumiya et al., 1991: Lipscombe et al., 1992), and consequently heterozygotes have low levels of MBL in the blood. Low levels of MBL have been linked to severe and recurrent infections in children (Summerfield et al., 1997). 36 J. N. Arnold et al. Figure 5. Structure of MBL. MBL is composed of identical 25kDa polypeptides that form a trimer through the formation of a triple helix of the collagen-like regions that is the basis of the MBL subunit (or monomer). This subunit can then disulphide bridge at its N-terminus to form higher order structures. MBL circulates in the serum mainly as a hexameric molecule (i.e. six subunits, 18 polypeptide chains). The collagen-like region is attached to a Carbohydrate Recognition Domain (CRD) which binds to sugar arrays that have hydroxyl groups on the carbon-3 and carbon-4 orientated in the equatorial plane of the pyranose ring (Weis et al., 1992). MBL participates in the host defense response through two major pathways. Firstly, it acts directly as an opsonin, promoting phagocytosis of foreign material to which it has bound. There are several candidate receptors through which this process may be mediated. The main candidate receptor is cell surface calreticulin (Sim et al., 1998; Ogden et al., 2001), but there is also evidence for the participation of complement receptor 1 (CR1: CD35) (Ghiran et al., 2000). The second pathway through which MBL functions is by triggering the lectin pathway of complement activation via MBL associated serine protease-2 (MASP-2) (Vorup-Jensen et al., 2000; Hajela et al., 2002). 3.2. MASPs MBL circulates in the serum bound to the serine protease pro-enzymes, MASPs, of which three have been identified to date; MASP-1, MASP-2 (Matsushita et al., 1992; Thiel et al., 1997) and MASP-3 (Dahl et al., 2001). The MBL-MASP complex was shown to be capable of consuming the complement components C2 and C4 (Ikeda et al., 1987). It is now generally accepted from recombinant protein work that MASP-2 is solely responsibly for the cleavage of C2 and C4 to produce C4b2a (Vorup-Jensen et al., 2000). This provides MASP-2 with a function similar to that of C1s in the C1 complex. The biological roles for MASP-1 and MASP-3 are currently unknown. MASP-1 has been shown to cleave ‘dead’ C3 (C3 in which the thiolester bond has hydrolyzed) at a slow rate. Cleavage of physiological ‘live’ C3 (C3 in which the thiolester bond is intact) occurs at a very slow rate, suggested to Human Immunoglobulin Glycosylation 37 Figure 6. The Complement System. The lectin and classical pathways rely on cleavage of complement protein C4, forming C4b, to which C2 binds and is cleaved, that leads to the formation of C4b2a, a C3 convertase that activates C3. C3 is cleaved into C3a and C3b, which is further cleaved to form the iC3b opsonin. Activation of C3 leads to the formation of the membrane attack complex which causes cell lysis. The alternative pathway relies on preformed C3b, or C3(H O) which forms spontaneously at a slow rate. C3b binds factor B, which is 2 cleaved by Factor D to form another C3 convertase, C3bBb. The C3 convertases are inactivated by decay accelerating factor, Factor H, C4b-binding protein and complement receptor I, which speed up the dissociation of the convertase. C3b and C4b when bound by cofactors such as Factor H are cleaved by Factor I and inactivated. The C3 convertases have naturally short half lives in the circulation. be too slow to be physiologically important (Hajela et al., 2002). MASP-1 also cleaves Factor XIII (plasma transglutaminase) and fibrinogen, two substrates of thrombin, potentially implicating MASP-1 in localized coagulation (Hajela et al., 2002). 3.3. Complement and the lectin pathway of complement activation The complement system (Fig. 6) is a major part of the innate immune response that eliminates foreign and altered-self cells by opsonisation and lysis. It is the body’s first line of defense against infectious agents. The complement system recognizes foreign matter through proteins with specific binding affinities to potential Pathogen Associated Molecular Patterns (PAMPs) including lipopolysaccharide, lipoproteins, peptidoglycan, lipoarabinomannan and oligosaccharide and charge arrays. The binding of ‘recognition’ proteins MBL and C1q leads to the activation of the complement cascade which is controlled and propagated through serine proteases and regulated directly by a serpin, C1-inhibitor (Cooper, 1985) that binds and inactivates these cascade triggering proteases. There are three routes of complement activation; the classical, alternative and lectin pathways. The classical pathway is triggered by the C1 complex. The C1 complex is composed of C1q and 2 each of the serine proteases C1r and C1s (Arlaud et al., 1987). 38 J. N. Arnold et al. Figure 7. MBL and the Immunoglobulins A summary of the interaction of MBL with the immunoglobulins. MBL is the recognition molecule of the lectin pathways of complement activation. Binding of MBL to a target activates MASPs. Activated MASP-2 cleaves the complement protein C4, forming C4b, to which C2 binds and is also cleaved by MASP-2, leading to the formation of C4b2a, a C3 convertase that activates C3. C3 is cleaved into C3a and C3b, which is further cleaved to form the iC3b opsonin. Activation of C3 leads on to the formation of the membrane attack complex (MAC) that causes cell lysis. 4. THE INTERACTION OF MBL WITH THE IMMUNOGLOBULINS The immunoglobulins contain populations of glycans, some of which terminate in mannose or GlcNAc which are potential binding ligands for lectin-like recognition proteins of the innate immune system, such as MBL, macrophage Mannose Receptor and the surfactant proteins SP-A and SP-D. The known interactions of MBL with immunoglobulins are summarised in Fig. 7. The glycans of IgG have restricted motion because of the terminal galactose residues attached to the glycan structures. The IgG CH2 domain has a hydrophobic area on the peptide surface of each heavy chain. Galactoses attached to the a1,6 arm of the glycan interact with this region, and this together with >80 other interactions such as hydrogen bonding and van der Waals interactions holds the glycan in contact with the protein surface, which also prevents further processing to attach terminal sialic acid (Wormald et al., 1997). The glycans therefore have limited Human Immunoglobulin Glycosylation 39 mobility. In IgG-G0, the glycans do not have terminal galactose residues, but terminal GlcNAc residues. These glycans are more mobile, as the glycan-protein interactions are not sufficient to hold the glycan anchored to the protein surface (Wormald et al., 1997). MBL has been shown to bind to the terminal GlcNAc residues of the IgG-G0 glycans (Malhotra et al., 1995). IgG-G0 glycoforms have been shown to increase dramatically in Rheumatoid Arthritis (RA) (Parekh et al., 1985). This increase has been shown to correlate with disease activity (Rook et al., 1991). Garred et al. (2000) correlated MBL levels with disease onset and progression in RA patients. This was consistent with the suggestion by Malhotra et al. (1995) that activating the lectin pathway of the complement system could be a potential route to additional inflammation in RA. IgD has the same domain structure as IgG, however the glycans found at the N-linked site homologous to that in IgG (Asn-297 in IgG and Asn-354 in IgD) are solely oligomannose structures. Although these are potential ligands for MBL, MBL does not bind IgD (Arnold et al., 2004). The oligomannose glycans at Asn-354 are inaccessible to MBL because the complex glycans occupying Asn-445 on the CH3 domain block the access to the oligomannose glycans (Arnold et al., 2004). MBL has been shown to interact with certain polymeric types of IgA but not SIgA (Roos et al., 2001; Royle et al., 2003). MBL binds to polymeric and dimeric forms of IgA with the highest avidity, but MBL does not bind to monomeric serum IgA (Roos et al., 2001). The glycans in IgA with which MBL is interacting have not been identified although it has been inferred from models that all the glycans on IgA (but not SIgA) are exposed and could potentially bind (Mattu et al., 1998). SIgA contains a large array of glycans terminating in GlcNAc residues (Royle et al., 2003). However these structures are masked from lectin binding by the SC which wraps around the IgA heavy chains. The SC itself contains predominantly sialylated complex glycans (Royle et al., 2003). The SC structure blocks access of MBL to the IgA glycans, although it has been suggested that these may be revealed when SC binds to pathogens (Royle et al., 2003). IgE has a different ff domain structure from IgG, IgD and IgA. The hinge peptides are replaced by immunoglobulin domains which form a rigid dimer. The crystal structure of the Fc and CH2 hinge domain showed an asymmetrically bent quaternary structure, where the CH2 domain bends over one side of the Fc (Wan et al., 2002). Oligomannose structures occupy Asn-394 (homologous site to Asn-297 in IgG and Asn-354 in IgD) (Dorrington and Bennich, 1978; Arnold et al., 2004). MBL, however, does not bind IgE (Arnold et al., 2004). The access to these oligomannose glycans is prevented because of the CH2 hinge domain which completely blocks access to the oligomannose glycans from one side. The CH2 hinge domain is proposed to ‘flip’ between two bent quaternary conformations with the CH2 hinge domains on either side of the Fc domain, preventing access to the oligomannose glycans from both sides of the Fc (Arnold et al., 2004). IgM is found in the serum as a pentamer and a hexamer. The IgM monomer unit has a very similar structure to that of IgE, with an Ig domain replacing the hinge region. IgM, however, contains oligomannose glycans at two N-linked glycosylation sites, located at Asn-402, homologous to the Asn-394 in IgE (and Asn-297 in IgG and Asn-354 in IgD) and at Asn-563 at the C-terminus (Wormald et al., 1991). It has been shown that immobilised human IgM does not bind MBL on microtitre 40 J. N. Arnold et al. plates (Roos et al., 2003). The oligomannose glycans at Asn-402 are predicted to be inaccessible on the basis that it is similar in structure to IgE, where the CH2 domain ‘flips,’ between two bent quaternary conformations (J. Arnold and M. Wormald, unpublished data). The accessibility of the tail piece oligomannose glycans is currently unknown and under investigation. It may be the case that the structural change that occurs upon IgM binding to antigen (referred to as the staple form of IgM), may present the oligomannose sugars to MBL for binding. There have been reports (see Fig. 7) of human IgM binding to rat MBL (Koppel and Solomon, 2001) and human, bovine and murine IgM binding to rabbit MBL (Nevens et al., 1992) (Fig. 7). In the latter case it appears that MBL may be binding only a small subpopulation of human IgM (J.Arnold, unpublished data). 5. CONCLUSIONS The glycans attached to the immunoglobulins have a great diversity in structure, location and number. The predominant complex glycan structures are biantennary, which are variably galactosylated and sialylated. There is also a high proportion of structures that contain either or both a bisecting GlcNAc and/or core fucose residue, in different ff percentages between the immunoglobulins. Glycan structures that could act as potential ligands for MBL have been identified on all the immunoglobulins. In human serum only IgG-G0 and polymeric and dimeric IgA have been shown to bind MBL and initiate the lectin pathway of complement (Malhotra et al., 1995; Roos et al., 2001). In other immunoglobulins small quantities of GlcNAc-terminating glycan structures have been identified in the glycan pool. These structures may define small subpopulations of the immunoglobulins to which MBL could bind. REFERENCES Amzel, L. M., and Poljak, R. J. (1979). Three-dimensional structure of immunoglobulins. Annu Rev Biochem 48, 961–997. Anton, L. C., Alcolea, J. M., Sanchez-Corral, P., Marques, G., Sanchez, A., and Vivanco, F. (1989). C3 binds covalently to the C gamma 3 domain of IgG immune aggregates during complement activation by the alternative pathway. Biochem J 257, 831–838. Arlaud, G. J., and Colomb, M. G. (1987). Modelling of C1, the first component of human complement: towards a consensus? Mol Immunol 24, 317. Arnold, J. N., Radcliffe, ff C. M., Wormald, M. R., Royle, L., Harvey, D. J., Crispin, M., Dwek, R. A., Sim, R. B., and Rudd, P. M. (2004) In Press J Immunol. Baenziger, J., and Kornfeld, S. (1974). Structure of the carbohydrate units of IgA1 immunoglobulin. I. Composition, glycopeptide isolation, and structure of the asparagines linked oligosaccharide units. J Biol Chem 249, 7260–7269. Baenziger, J., Kornfeld, S., and Kochwa, S. (1974). Structure of the carbohydrate units of IgE immunoglobulin. I. Over-all composition, glycopeptide isolation, and structure of the high mannose oligosaccharide unit. J Biol Chem 249, 1889–1896. Baenziger, J., Kornfeld, S., and Kochwa, S. (1974). Structure of the carbohydrate units of IgE immunoglobulin. II. Sequence of the sialic acid-containing glycopeptides. J Biol Chem 249, 1897–1903. Bos, N. A., Bun, J. C., Popma, S. H., Cebra, E. R., Deenen, G. J., van der Cammen, M. J., Kroese, F. G., and Cebra, J. J. (1996). Monoclonal immunoglobulin A derived from peritoneal B cells is encoded by Human Immunoglobulin Glycosylation 41 both germ line and somatically mutated VH genes and is reactive with commensal bacteria. Infect Immun 64, 616–623. Butler, M., Quelhas, D., Critchley, A. J., Carchon, H., Hebestreit, H. F., Hibbert, R. G., Vilarinho, L., Teles, E., Matthijs, G., Schollen, E., et al. (2003). Detailed glycan analysis of serum glycoproteins of patients with congenital disorders of glycosylation indicates the specific defective glycan processing step and provides an insight into pathogenesis. Glycobiology 13, 601–622. Chapman, A., and Kornfeld, R. (1979). Structure of the high mannose oligosaccharides of a human IgM myeloma protein. II. The minor oligosaccharides of high mannose glycopeptide. J Biol Chem 254, 824–828. Cooper, N. R. (1985). The classical complement pathway: activation and regulation of the first complement component. Adv Immunol 37, 151–216. Crispin, M. D., Ritchie, G. E., Critchley, A. J., Morgan, B. P., Wilson, I. A., Dwek, R. A., Sim, R. B., and Rudd, P. M. (2004). Monoglucosylated glycans in the secreted human complement component C3: implications for protein biosynthesis and structure. FEBS Lett 566, 270–274. Dahl, M. R., Thiel, S., Matsushita, M., Fujita, T., Willis, A. C., Christensen, T., Vorup-Jensen, T., and Jensenius, J. C. (2001). MASP-3 and its association with distinct complexes of the mannan-binding lectin complement activation pathway. Immunity 15, 127–135. Delacroix, D. L., Dive, C., Rambaud, J. C., and Vaerman, J. P. (1982). IgA subclasses in various secretions and in serum. Immunology 47, 383–385. Dorrington, K. J., and Bennich, H. H. (1978). Structure-function relationships in human immunoglobulin E. Immunol Rev 41, 3–25. Duncan, A. R., and Winter, G. (1988). The binding site for C1q on IgG. Nature 332, 738–740. Endo, T., Mestecky, J., Kulhavy, R., and Kobata, A. (1994). Carbohydrate heterogeneity of human myeloma proteins of the IgA1 and IgA2 subclasses. Mol Immunol 31, 1415–1422. Field, M. C., Amatayakul-Chantler, S., Rademacher, T. W., Rudd, P. M., and Dwek, R. A. (1994). Structural analysis of the N-glycans from human immunoglobulin A1: comparison of normal human serum immunoglobulin A1 with that isolated from patients with rheumatoid arthritis. Biochem J 299 (Pt 1), 261–275. Gala, F. A., and Morrison, S. L. (2002). The role of constant region carbohydrate in the assembly and secretion of human IgD and IgA1. J Biol Chem 277, 29005–29011. Garred, P., Madsen, H. O., Marquart, H., Hansen, T. M., Sorensen, S. F., Petersen, J., Volck, B., Svejgaard, A., Graudal, N. A., Rudd, P. M., et al. (2000). Two edged role of mannose binding lectin in rheumatoid arthritis: a cross sectional study. J Rheumatol 27, 26–34. Ghiran, I., Barbashov, S. F., Klickstein, L. B., Tas, S. W., Jensenius, J. C., and Nicholson-Weller, A. (2000). Complement receptor 1/CD35 is a receptor for mannan-binding lectin. J Exp Med 192, 1797–1808. ff B. E., Ferluga, J., Hajela, S., Gal, P., and Sim, Hajela, K., Kojima, M., Ambrus, G., Wong, K. H., Moffatt, R. B. (2002). The biological functions of MBL-associated serine proteases (MASPs). Immunobiology 205, 467–475. Holmskov, U., Malhotra, R., Sim, R. B., and Jensenius, J. C. (1994). Collectins: collagenous C- type lectins of the innate immune defense system. Immunol Today 15, 67–74. Ikeda, K., Sannoh, T., Kawasaki, N., Kawasaki, T., and Yamashina, I. (1987). Serum lectin with known structure activates complement through the classical pathway. J Biol Chem 262, 7451–7454. Iobst, S. T., and Drickamer, K. (1994). Binding of sugar ligands to Ca(2+)-dependent animal lectins. II. Generation of high-affinity galactose binding by site-directed mutagenesis. J Biol Chem 269, 15512–15519. Jefferis, ff R., Lund, J., Mizutani, H., Nakagawa, H., Kawazoe, Y., Arata, Y., and Takahashi, N. (1990). A comparative study of the N-linked oligosaccharide structures of human IgG subclass proteins. Biochem J 268, 529–537. Johansen, F. E., Braathen, R., and Brandtzaeg, P. (2001). The J chain is essential for polymeric Ig receptormediated epithelial transport of IgA. J Immunol 167, 5185–5192. Koppel, R., and Solomon, B. (2001). IgM detection via selective recognition by mannose-binding protein. J Biochem Biophys Methods 49, 641–647. Lamm, M. E. (1997). Interaction of antigens and antibodies at mucosal surfaces. Annu Rev Microbiol 51, 311–340. Lipscombe, R. J., Sumiya, M., Hill, A. V., Lau, Y. L., Levinsky, R. J., Summerfield, J. A., and Turner, M. W. (1992). High frequencies in African and non-African populations of independent mutations in the mannose binding protein gene. Hum Mol Genet 1, 709–715. 42 J. N. Arnold et al. Litman, G. W., Anderson, M. K., and Rast, J. P. (1999). Evolution of antigen binding receptors. Annu Rev Immunol 17, 109–147. ff molecular events Madsen, H. O., Satz, M. L., Hogh, B., Svejgaard, A., and Garred, P. (1998). Different result in low protein levels of mannan-binding lectin in populations from southeast Africa and South America. J Immunol 161, 3169–3175. Malhotra, R., Lu, J., Holmskov, U., and Sim, R. B. (1994). Collectins, collectin receptors and the lectin pathway of complement activation. Clin Exp Immunol 97 Suppl 2, 4–9. Malhotra, R., Wormald, M. R., Rudd, P. M., Fischer, P. B., Dwek, R. A., and Sim, R. B. (1995). Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nat Med 1, 237–243. Matsushita, M., and Fujita, T. (1992). Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. J Exp Med 176, 1497–1502. Mattu, T. S., Pleass, R. J., Willis, A. C., Kilian, M., Wormald, M. R., Lellouch, A. C., Rudd, P. M., Woof, J. M., and Dwek, R. A. (1998). The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fc alpha receptor interactions. J Biol Chem 273, 2260–2272. Mellis, S. J., and Baenziger, J. U. (1983). Structures of the O-glycosidically linked oligosaccharides of human IgD. J Biol Chem 258, 11557–11563. Mellis, S. J., and Baenziger, J. U. (1983). Structures of the oligosaccharides present at the three asparaginelinked glycosylation sites of human IgD. J Biol Chem 258, 11546–11556. ff R. Mimura, Y., Church, S., Ghirlando, R., Ashton, P. R., Dong, S., Goodall, M., Lund, J., and Jefferis, (2000). The influence of glycosylation on the thermal stability and effector ff function expression of human IgG1-Fc: properties of a series of truncated glycoforms. Mol Immunol 37, 697–706. Nevens, J. R., Mallia, A. K., Wendt, M. W., and Smith, P. K. (1992). Affinity chromatographic purification of immunoglobulin M antibodies utilizing immobilized mannan binding protein. J Chromatogr 597, 247–256. Norderhaug, I. N., Johansen, F. E., Schjerven, H., and Brandtzaeg, P. (1999). Regulation of the formation and external transport of secretory immunoglobulins. Crit Rev Immunol 19, 481– 508. Ogden, C. A., deCathelineau, A., Hoffmann, ff P. R., Bratton, D., Ghebrehiwet, B., Fadok, V. A., and Henson, P. M. (2001). C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med 194, 781–795. Parekh, R. B., Dwek, R. A., Sutton, B. J., Fernandes, D. L., Leung, A., Stanworth, D., Rademacher, T. W., Mizuochi, T., Taniguchi, T., Matsuta, K., and et al. (1985). Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature 316, 452–457. Rook, G. A., Steele, J., Brealey, R., Whyte, A., Isenberg, D., Sumar, N., Nelson, J. L., Bodman, K. B., Young, A., Roitt, I. M., and et al. (1991). Changes in IgG glycoform levels are associated with remission of arthritis during pregnancy. J Autoimmun 4, 779–794. Roos, A., Bouwman, L. H., Munoz, J., Zuiverloon, T., Faber-Krol, M. C., Fallaux-van den Houten, F. C., Klar-Mohamad, N., Hack, C. E., Tilanus, M. G., and Daha, M. R. (2003). Functional characterization of the lectin pathway of complement in human serum. Mol Immunol 39, 655–668. Roos, A., Bouwman, L. H., van Gijlswijk-Janssen, D. J., Faber-Krol, M. C., Stahl, G. L., and Daha, M. R. (2001). Human IgA activates the complement system via the mannan-binding lectin pathway. J Immunol 167, 2861–2868. Royle, L., Roos, A., Harvey, D. J., Wormald, M. R., van Gijlswijk-Janssen, D., Redwan el, R. M., Wilson, I. A., Daha, M. R., Dwek, R. A., and Rudd, P. M. (2003). Secretory IgA N- and O- glycans provide a link between the innate and adaptive immune systems. J Biol Chem 278, 20140–20153. Rudd, P. M., Fortune, F., Patel, T., Parekh, R. B., Dwek, R. A., and Lehner, T. (1994). A human T-cell receptor recognizes ‘O’-linked sugars from the hinge region of human IgA1 and IgD. Immunology 83, 99–106. Sarmay, G., Lund, J., Rozsnyay, Z., Gergely, J., and Jefferis, ff R. (1992). Mapping and comparison of the interaction sites on the Fc region of IgG responsible for triggering antibody dependent cellular cytotoxicity (ADCC) through different ff types of human Fc gamma receptor. Mol Immunol 29, 633–639. Shields, R. L., Namenuk, A. K., Hong, K., Meng, Y. G., Rae, J., Briggs, J., Xie, D., Lai, J., Stadlen, A., Li, B., Human Immunoglobulin Glycosylation 43 et al. (2001). High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J Biol Chem 276, 6591–6604. Sim, R. B., Moestrup, S. K., Stuart, G. R., Lynch, N. J., Lu, J., Schwaeble, W. J., and Malhotra, R. (1998). Interaction of C1q and the collectins with the potential receptors calreticulin (cC1qR/collectin receptor) and megalin. Immunobiology 199, 208–224. Sorensen, V., Sundvold, V., Michaelsen, T. E., and Sandlie, I. (1999). Polymerization of IgA and IgM: roles of Cys309/Cys414 and the secretory tailpiece. J Immunol 162, 3448–3455. Stockert, R. J. (1995). The asialoglycoprotein receptor: relationships between structure, function, and expression. Physiol Rev 75, 591–609. Sumiya, M., Super, M., Tabona, P., Levinsky, R. J., Arai, T., Turner, M. W., and Summerfield, J. A. (1991). Molecular basis of opsonic defect in immunodeficient children. Lancet 337, 1569– 1570. Summerfield, J. A., Sumiya, M., Levin, M., and Turner, M. W. (1997). Association of mutations in mannose binding protein gene with childhood infection in consecutive hospital series. Bmj 314, 1229–1232. Takahashi, N., Tetaert, D., Debuire, B., Lin, L. C., and Putnam, F. W. (1982). Complete amino acid sequence of the delta heavy chain of human immunoglobulin D. Proc Natl Acad Sci U S A 79, 2850–2854. Takayasu, T., Suzuki, S., Kametani, F., Takahashi, N., Shinoda, T., Okuyama, T., and Munekata, E. (1982). Amino acid sequence of galactosamine-containing glycopeptides in the hinge region of a human immunoglobulin D. Biochem Biophys Res Commun 105, 1066–1071. Tarelli, E., Smith, A. C., Hendry, B. M., Challacombe, S. J., and Pouria, S. (2004). Human serum IgA1 is substituted with up to six O-glycans as shown by matrix assisted laser desorption ionisation time-offlight mass spectrometry. Carbohydr Res 339, 2329–2335. Tarentino, A. L., Plummer, T. H., Jr., and Maley, F. (1974). The release of intact oligosaccharides from specific glycoproteins by endo-beta-N-acetylglucosaminidase H. J Biol Chem 249, 818– 824. Thiel, S., Vorup-Jensen, T., Stover, C. M., Schwaeble, W., Laursen, S. B., Poulsen, K., Willis, A. C., Eggleton, P., Hansen, S., Holmskov, U., et al. (1997). A second serine protease associated with mannan-binding lectin that activates complement. Nature 386, 506–510. Turner, M. W. (1996). Mannose-binding lectin: the pluripotent molecule of the innate immune system. Immunol Today 17, 532–540. Valdimarsson, H., Stefansson, M., Vikingsdottir, T., Arason, G. J., Koch, C., Thiel, S., and Jensenius, J. C. (1998). Reconstitution of opsonizing activity by infusion of mannan-binding lectin (MBL) to MBLdeficient humans. Scand J Immunol 48, 116–123. Van Boxel, J. A., Paul, W. E., Terry, W. D., and Green, I. (1972). Communications. IgD-bearing human lymphocytes. J Immunol 109, 648–651. Vorup-Jensen, T., Petersen, S. V., Hansen, A. G., Poulsen, K., Schwaeble, W., Sim, R. B., Reid, K. B., Davis, S. J., Thiel, S., and Jensenius, J. C. (2000). Distinct pathways of mannan- binding lectin (MBL)- and C1-complex autoactivation revealed by reconstitution of MBL with recombinant MBL-associated serine protease-2. J Immunol 165, 2093–2100. Wan, T., Beavil, R. L., Fabiane, S. M., Beavil, A. J., Sohi, M. K., Keown, M., Young, R. J., Henry, A. J., Owens, R. J., Gould, H. J., and Sutton, B. J. (2002). The crystal structure of IgE Fc reveals an asymmetrically bent conformation. Nat Immunol 3, 681–686. Weis, W. I., Drickamer, K., and Hendrickson, W. A. (1992). Structure of a C-type mannose- binding protein complexed with an oligosaccharide. Nature 360, 127–134. Wiersma, E. J., Collins, C., Fazel, S., and Shulman, M. J. (1998). Structural and functional analysis of J chain-deficient IgM. J Immunol 160, 5979–5989. Wormald, M. R., Rudd, P. M., Harvey, D. J., Chang, S. C., Scragg, I. G., and Dwek, R. A. (1997). Variations in oligosaccharide-protein interactions in immunoglobulin G determine the site-specific glycosylation profiles and modulate the dynamic motion of the Fc oligosaccharides. Biochemistry 36, 1370–1380. Wormald, M. R., Wooten, E. W., Bazzo, R., Edge, C. J., Feinstein, A., Rademacher, T. W., and Dwek, R. A. (1991). The conformational effects ff of N-glycosylation on the tailpiece from serum IgM. Eur J Biochem 198, 131–139. Youings, A., Chang, S. C., Dwek, R. A., and Scragg, I. G. (1996). Site-specific glycosylation of human immunoglobulin G is altered in four rheumatoid arthritis patients. Biochem J 314 (Pt 2), 621–630. Zhu, D., McCarthy, H., Ottensmeier, C. H., Johnson, P., Hamblin, T. J., and Stevenson, F. K. (2002). Acquisition of potential N-glycosylation sites in the immunoglobulin variable region by somatic mutation is a distinctive feature of follicular lymphoma. Blood 99, 2562–2568.