Synthesis of Anhydrides and Derivatization of Glycans for Better
advertisement

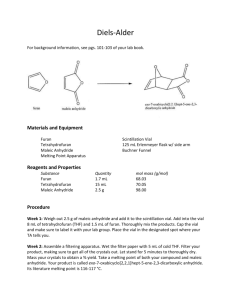
Synthesis of Anhydrides and Derivatization of Glycans for Better Elucidation of Molecular Structure Through NMR Spectroscopy Solongo Batjargal Mentor: Athan Shaka Glycans play essential roles in cell-cell interactions and in many other biological phenomena. The malfunctions of these glycans are often involved in human diseases. The development of a method to determine the structure of complex glycans would have widespread applications in medicine. Unfortunately, identifying the structures of glycans using NMR spectroscopy is challenging due to their types of linkages, stereoisomers, and similar functional groups. Acetylation of glycans was achieved in a previous project using acetic anhydride with a catalyst, scandium triflate, to reduce the spectral crowding. The overlapping 1H signals can spread out even more by adding more electron-withdrawing groups such as α,α-difluoroacetic anhydride, and α,α-dichloropropionic anhydride to the glycans. Developed by a graduate student in my group, a new pulse sequence called Selective Heteronuclear Hartman-Hahn (SHEHAHA), which requires 13C-isotag, allows us to identify glycans unequivocally. I have explored methods to synthesize 13C-anhydrides that are not commercially available. Dehydrating agent phosphorous pentoxide, P2O5 and polymer supported dicyclohexylcarbodiimide column (PS-DCC) were used to synthesize difluoroacetic anhydrides and dichloropropionic anhydride. P2O5 successfully synthesized clean anhydrides with a high yield; meanwhile the yield of anhydrides synthesized by PS-DCC was low. The NMR spectrum of difluoroacetic anhydride shows a triplet of proton signal at 6.02 ppm with a coupling constant of 55Hz. This anhydride can derivatize the glycans to spread out the signals of peaks.