Vibrational distribution of Br2 molecule following photodissociation
advertisement
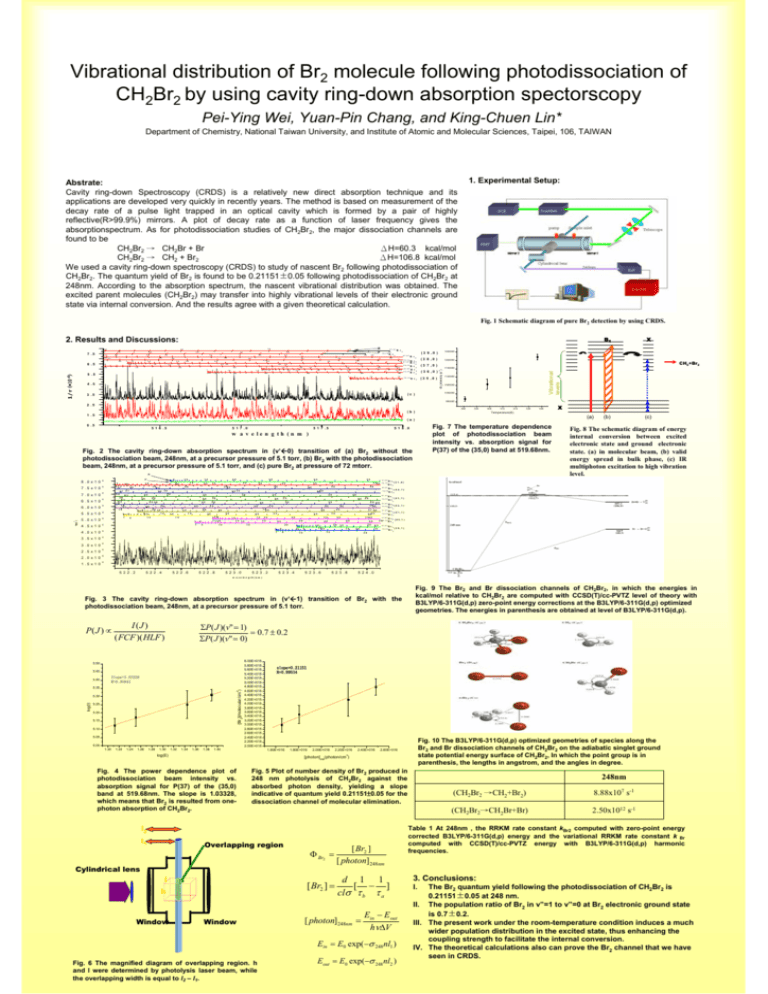
Vibrational distribution of Br2 molecule following photodissociation of CH2Br2 by using cavity ring-down absorption spectorscopy Pei-Ying Wei, Yuan-Pin Chang, and King-Chuen Lin* Department of Chemistry, National Taiwan University, and Institute of Atomic and Molecular Sciences, Taipei, 106, TAIWAN 1. Experimental Setup: Abstrate: Cavity ring-down Spectroscopy (CRDS) is a relatively new direct absorption technique and its applications are developed very quickly in recently years. The method is based on measurement of the decay rate of a pulse light trapped in an optical cavity which is formed by a pair of highly reflective(R>99.9%) mirrors. A plot of decay rate as a function of laser frequency gives the absorptionspectrum. As for photodissociation studies of CH2Br2, the major dissociation channels are found to be CH2Br2 → CH2Br + Br ΔH=60.3 kcal/mol CH2Br2 → CH2 + Br2 ΔH=106.8 kcal/mol We used a cavity ring-down spectroscopy (CRDS) to study of nascent Br2 following photodissociation of CH2Br2. The quantum yield of Br2 is found to be 0.21151±0.05 following photodissociation of CH2Br2 at 248nm. According to the absorption spectrum, the nascent vibrational distribution was obtained. The excited parent molecules (CH2Br2) may transfer into highly vibrational levels of their electronic ground state via internal conversion. And the results agree with a given theoretical calculation. Fig. 1 Schematic diagram of pure Br2 detection by using CRDS. B1 2. Results and Discussions: 5 1 1 5 3 0 3 0 1 0 5 1 0 1 0 5 1 5 5 3 0 1 0 8 1 ,8 1 4 0 3 5 R P R P 2 5 1 5 R P 2 5 7 9 ,7 9 8 1 ,8 1 7 9 ,7 9 8 1 ,8 1 2 9 2 4 7 9 ,7 9 2 8 1 0 1 2 P 2 0 2 0 B r 2 R 3 5 2 5 1 5 B r R P 3 5 2 0 1 5 2 0 1 5 8 1 ,8 1 4 5 7 9 ,7 9 4 0 4 0 3 0 3 0 1 0 5 1 1 0 4 0 3 0 2 5 1 2 5 5 1 2 5 2 0 2 0 1 1/τ(*10-6) 3 5 3 5 2 5 1 5 2 0 3 5 2 0 3 0 1 5 1 0 1 5 4 5 R P 1 5 1 0 5 7 9 ,7 9 1 4 B r 2 B r2 B r2 B r2 B r2 B r2 B r 2 4 .5 1250000 ( 3 8 ,0 ) 1200000 X CH2+Br2 ( 3 7 ,0 ) 1150000 ( 3 6 ,0 ) (3 5 ,0 ) 1100000 1050000 1000000 (c ) 3 .5 ( 3 9 ,0 ) Vibrational levels 1 1 4 5 .5 R P -1 2 5 2 5 2 5 6 .5 P 4 0 3 5 4 0 3 0 3 5 R P R 4 0 3 5 3 5 2 5 3 2 Br2intensity (s ) 3 0 2 1 2 0 4 0 3 5 3 0 7 .5 950000 2 .5 295 300 (b ) 305 310 315 320 325 (a) (a ) 0 .5 5 1 6 .5 5 1 7 .0 5 1 7 .5 Fig. 7 The temperature dependence plot of photodissociation beam intensity vs. absorption signal for P(37) of the (35,0) band at 519.68nm. 5 1 8 .0 w a v e le n g th (n m ) Fig. 2 The cavity ring-down absorption spectrum in (v’Å0) transition of (a) Br2 without the photodissociation beam, 248nm, at a precursor pressure of 5.1 torr, (b) Br2 with the photodissociation beam, 248nm, at a precursor pressure of 5.1 torr, and (c) pure Br2 at pressure of 72 mtorr. -1 1/τ(s ) R P 8 .0 x 1 0 6 7 .5 x 1 0 6 5 27 7 .0 x 1 0 6 26 7 6 .5 x 1 0 6 6 .0 x 1 0 6 5 .5 x 1 0 6 5 .0 x 1 0 6 4 .5 x 1 0 6 4 .0 x 1 0 6 3 .5 x 1 0 6 3 .0 x 1 0 6 2 .5 x 1 0 6 2 .0 x 1 0 6 1 .5 x 1 0 6 1 27 1 1 15 10 25 1 5 10 20 20 1 2 05 1 1 5 15 10 15 5 2 2 .6 5 2 2 .8 20 20 5 2 3 .0 5 2 3 .2 5 5 2 3 .4 15 8 1 ,8 1 B r2 7 9 ,7 9 B r2 20 5 2 3 .8 ( 4 1 ,1 ) B r2 8 1 ,8 1 20 19 20 7 9 ,7 9 B r2 B r2 ( 4 0 ,1 ) 2 87 9 , 7 9 B r 2 29 28 15 15 5 2 3 .6 8 1 ,8 1 3 43 3 25 25 10 1 01 5 (4 3 ,1 ) B r2 B r2 (4 2 ,1 ) B r2 7 9 ,7 9 (c) (3 1 ,0 ) 8 1 ,8 1 3 73 8 (b) Fig. 8 The schematic diagram of energy internal conversion between excited electronic state and ground electronic state. (a) in molecular beam, (b) valid energy spread in bulk phase, (c) IR multiphoton excitation to high vibration level. B r 2 (4 4 ,1 ) B r2 B r2 7 9 ,7 9 37 34 3 32 8 25 25 1 5 1 5 10 10 5 1 5 2 2 .4 30 30 20 20 1 5 2 2 .2 35 30 25 15 15 10 40 38 35 35 30 25 25 10 1 1 05 5 40 35 30 3 02 5 B r2 B r2 7 9 ,7 9 8 1 ,8 1 40 35 30 30 20 8 1 ,8 1 7 9 ,7 9 8 1 ,8 1 40 35 35 30 15 38 40 35 25 25 15 15 10 5 35 25 15 3 44 0 35 40 4 04 0 30 25 20 20 10 51 0 35 30 35 30 30 30 25 20 5 30 25 30 3535 25 20 1 25 20 2 53 5 30 30 25 20 15 25 20 30 23 22 23 22 15 15 17 16 10 5 20 1 53 0 10 27 5 1 15 10 X Temperature(K) 1 .5 (3 9 ,1 ) 5 2 4 .0 w a v e le n g t h ( n m ) Fig. 3 The cavity ring-down absorption spectrum in (v’Å1) transition of Br2 with the photodissociation beam, 248nm, at a precursor pressure of 5.1 torr. P( J ) ∝ ΣP ( J )(v" = 1) = 0.7 ± 0.2 ΣP ( J )(v" = 0) I (J ) ( FCF )( HLF ) 5.50 5.45 5.40 Slope=1.03328 R=0.99841 3 [Br2](molecular/cm ) 5.35 log(I) 5.30 5.25 5.20 5.15 5.10 5.05 5.00 1.20 1.22 1.24 1.26 1.28 1.30 1.32 1.34 1.36 1.38 6.00E+015 5.80E+015 5.60E+015 5.40E+015 5.20E+015 5.00E+015 4.80E+015 4.60E+015 4.40E+015 4.20E+015 4.00E+015 3.80E+015 3.60E+015 3.40E+015 3.20E+015 3.00E+015 2.80E+015 2.60E+015 2.40E+015 2.20E+015 2.00E+015 1.40 slope=0.21151 R=0.99514 1.60E+016 log(E) 1.80E+016 2.00E+016 2.20E+016 2.40E+016 Fig. 5 Plot of number density of Br2 produced in 248 nm photolysis of CH2Br2 against the absorbed photon density, yielding a slope indicative of quantum yield 0.21151±0.05 for the dissociation channel of molecular elimination. l2 Overlapping region Φ Br2 = Cylindrical lens l [ Br2 ] = h Window Fig. 10 The B3LYP/6-311G(d,p) optimized geometries of species along the Br2 and Br dissociation channels of CH2Br2 on the adiabatic singlet ground state potential energy surface of CH2Br2, In which the point group is in parenthesis, the lengths in angstrom, and the angles in degree. 2.60E+016 3 [photon]248(photon/cm ) Fig. 4 The power dependence plot of photodissociation beam intensity vs. absorption signal for P(37) of the (35,0) band at 519.68nm. The slope is 1.03328, which means that Br2 is resulted from onephoton absorption of CH2Br2. l1 Fig. 9 The Br2 and Br dissociation channels of CH2Br2, in which the energies in kcal/mol relative to CH2Br2 are computed with CCSD(T)/cc-PVTZ level of theory with B3LYP/6-311G(d,p) zero-point energy corrections at the B3LYP/6-311G(d,p) optimized geometries. The energies in parenthesis are obtained at level of B3LYP/6-311G(d,p). Window [ Br2 ] [ photon]248nm d 1 1 [ − ] clσ τ b τ a [ photon]248nm = Ein − Eout hνΔV Ein = E0 exp(−σ 248 nl1 ) Fig. 6 The magnified diagram of overlapping region. h and l were determined by photolysis laser beam, while the overlapping width is equal to l2 – l1. Eout = E0 exp(−σ 248 nl2 ) 248nm (CH2Br2 →CH2+Br2) 8.88x107 s-1 (CH2Br2→CH2Br+Br) 2.50x1012 s-1 Table 1 At 248nm , the RRKM rate constant kBr2 computed with zero-point energy corrected B3LYP/6-311G(d,p) energy and the variational RRKM rate constant k Br computed with CCSD(T)/cc-PVTZ energy with B3LYP/6-311G(d,p) harmonic frequencies. 3. Conclusions: I. The Br2 quantum yield following the photodissociation of CH2Br2 is 0.21151±0.05 at 248 nm. II. The population ratio of Br2 in v”=1 to v”=0 at Br2 electronic ground state is 0.7±0.2. III. The present work under the room-temperature condition induces a much wider population distribution in the excited state, thus enhancing the coupling strength to facilitate the internal conversion. IV. The theoretical calculations also can prove the Br2 channel that we have seen in CRDS.