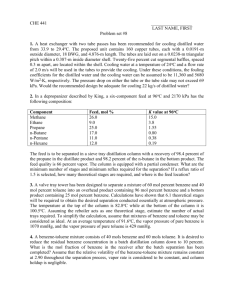
Benzene Toluene Naphthalene Examples of Aromatic
advertisement

Examples of Aromatic Hydrocarbons CH3 H H H H H H H H H H Benzene H H Toluene H H H H H H H Naphthalene Some history 11.1 Benzene 1825 Michael Faraday isolates a new hydrocarbon from illuminating gas. 1834 Eilhardt Mitscherlich isolates same substance and determines its empirical formula to be CnHn. Compound comes to be called benzene. 1845 August W. von Hofmann isolates benzene from coal tar. 1866 August Kekulé proposes structure of benzene. Kekulé Formulation of Benzene Kekulé proposed a cyclic structure for C6H6 with alternating single and double bonds. H 11.2 The Structure of Benzene H H H H H Kekulé Formulation of Benzene Kekulé Formulation of Benzene Later, Kekulé revised his proposal by suggesting a rapid equilibrium between two equivalent structures. H H H H H H H H H H H H However, this proposal suggested isomers of the kind shown were possible. Yet, none were ever found. X X H X H X H H H H H H Structure of Benzene All C—C bond distances = 140 pm Structural studies of benzene do not support the Kekulé formulation. Instead of alternating single and double bonds, all of the C—C bonds are the same length. 140 pm 140 pm 140 pm 140 pm 140 pm 140 pm is the average between the C—C single bond distance and the double bond distance in 1,3-butadiene. Benzene has the shape of a regular hexagon. Resonance Formulation of Benzene Kekulé Formulation of Benzene Instead of Kekulé's suggestion of a rapid equilibrium between two structures: H 140 pm express the structure of benzene as a resonance hybrid of the two Lewis structures. Electrons are not localized in alternating single and double bonds, but are delocalized over all six ring carbons. H H H H H H H H H H H H H H H H H H H H H H H Resonance Formulation of Benzene The Stability of Benzene Circle-in-a-ring notation stands for resonance description of benzene (hybrid of two Kekulé structures) Thermochemical Measures of Stability 3 x cyclohexene heat of hydrogenation: compare experimental value with "expected" value for hypothetical "cyclohexatriene" + 3H2 ”Expected" heat of hydrogenation of benzene is 3 x heat of hydrogenation of cyclohexene. Pt 120 kJ/mol ΔH°= – 208 kJ 360 kJ/mol 3 x cyclohexene Observed heat of hydrogenation is 152 kJ/mol less than "expected." Benzene is 152 kJ/mol more stable than expected; 152 kJ/mol is the resonance energy of benzene. 360 kJ/mol 208 kJ/mol Hydrogenation of 1,3cyclohexadiene (+2H2) gives off more heat than hydrogenation of benzene (+3H2)! 231 kJ/mol 208 kJ/mol 3 x cyclohexene Cyclic conjugation versus noncyclic conjugation 3H2 Pt 360 kJ/mol 231 kJ/mol 120 kJ/mol 208 kJ/mol heat of hydrogenation = 208 kJ/mol 3H2 Pt heat of hydrogenation = 337 kJ/mol Resonance Energy of Benzene compared to localized 1,3,5-cyclohexatriene 152 kJ/mol compared to 1,3,5-hexatriene 129 kJ/mol 11.4 An Orbital Hybridization View of Bonding in Benzene Exact value of resonance energy of benzene depends on what it is compared to, but regardless of model, benzene is more stable than expected by a substantial amount. Orbital Hybridization Model of Bonding in Benzene Planar ring of 6 sp2 hybridized carbons Orbital Hybridization Model of Bonding in Benzene Each carbon contributes a p orbital Six p orbitals overlap to give cyclic π system; six π electrons delocalized throughout π system Orbital Hybridization Model of Bonding in Benzene High electron density above and below plane of ring The π Molecular Orbitals of Benzene Benzene MOs Benzene MOs Antibonding orbitals Energy Antibonding orbitals Energy Bonding orbitals 6 p AOs combine to give 6 π MOs 3 MOs are bonding; 3 are antibonding Bonding orbitals All bonding MOs are filled No electrons in antibonding orbitals Benzene MOs Figure 11.4 p 435 General Points General Points 1) Benzene is considered as the parent and comes last in the name. Br C(CH3)3 NO2 1) Benzene is considered as the parent and comes last in the name. 2) List substituents in alphabetical order. 3) Number ring in direction that gives lowest locant at first point of difference. Bromobenzene tert-Butylbenzene Nitrobenzene Example Ortho, Meta, and Para Alternative locants for disubstituted derivatives of benzene Cl Br F 1,2 = ortho (abbreviated o-) 2-bromo-1-chloro-4-fluorobenzene 1,3 = meta (abbreviated m-) Examples NO2 Cl CH2CH3 O CH Cl o-ethylnitrobenzene m-dichlorobenzene (1-ethyl-2-nitrobenzene) (1,3-dichlorobenzene) Benzaldehyde 1,4 = para (abbreviated p-) O CH COH Styrene Benzoic acid O OH CCH3 Acetophenone Phenol CH2 NH2 OCH3 Aniline Anisole Names in Can be Used as Parent OCH3 Easily Confused Names OCH3 phenyl phenol benzyl OH CH2— NO2 Anisole p-Nitroanisole or 4-Nitroanisole a group a compound a group Naphthalene Resonance energy = 255 kJ/mol Polycyclic Aromatic Hydrocarbons Most stable Lewis structure; both rings correspond to Kekulé benzene. Anthracene and Phenanthrene Physical Properties of Arenes Anthracene Phenanthrene resonance energy: 347 kJ/mol 381 kJ/mol Physical Properties Arenes (aromatic hydrocarbons) resemble other hydrocarbons. They are: nonpolar insoluble in water less dense than water Reduction of Benzene Rings 1. Reactions involving the ring H a) Reduction Catalytic hydrogenation (Section 11.3) Birch reduction (Section 11.10) b) Electrophilic aromatic substitution (Chapter 12) c) Nucleophilic aromatic substitution (Chapter 12) 2. The ring as a substituent (Sections 11.11-11.16) catalytic hydrogenation H H H H H H H H H H H H H H H H H Birch reduction H H H H H H H H Birch Reduction of Benzene H H H H H Na, NH3 H H H H CH3OH H H The Birch Reduction H H H (80%) Product is non-conjugated diene. Reaction stops here. There is no further reduction. Reaction is not hydrogenation. H2 is not involved in any way. Mechanism of the Birch Reduction Mechanism of the Birch Reduction Step 2: Proton transfer from methanol Step 1: Electron transfer from sodium H H H + • Na H H H H H H H • H •• – H H H + H • H H H H Na+ H H H – •• •• OCH3 •• • H •• – H H H •• OCH3 •• Mechanism of the Birch Reduction Mechanism of the Birch Reduction Step 3: Electron transfer from sodium H H H • H H Step 4: Proton transfer from methanol H + • Na H H H H – H H + •• H Birch Reduction of an Alkylbenzene H H H H H Na, NH3 H H H CH3OH C(CH3)3 H C(CH3)3 H H H H H H H H H H (86%) If an alkyl group is present on the ring, it ends up as a substituent on the double bond. H •• •• OCH3 H – Na+ H H H – •• •• OCH •• 3 H H •• H H H The Benzene Ring as a Substituent Bond-dissociation energy for C—H bond is equal to ΔH° for: C R—H C C• C• allylic radical Benzylic carbon is analogous to allylic carbon. Bond-dissociation Energies of Propene and Toluene H 2C CH C H 368 kJ/mol -H• H CH C H H 356 kJ/mol -H• Resonance in Benzyl Radical H C• H •H The more stable the free radical R•, the weaker the bond, and the smaller the bond-dissociation energy. H H 2C + and is about 400 kJ/mol for alkanes. benzylic radical H R• • C H H H H H H H C• H H Low BDEs indicate allyl and benzyl radical are more stable than simple alkyl radicals. Unpaired electron is delocalized between benzylic carbon and the ring carbons that are ortho and para to it. Resonance in Benzyl Radical H H C Resonance in Benzyl Radical H H • H H H H H C H H • H H H Unpaired electron is delocalized between benzylic carbon and the ring carbons that are ortho and para to it. Unpaired electron is delocalized between benzylic carbon and the ring carbons that are ortho and para to it. Resonance in Benzyl Radical Free-radical Chlorination of Toluene H H C H • H CH3 H H H Unpaired electron is delocalized between benzylic carbon and the ring carbons that are ortho and para to it. Toluene Cl2 CH2Cl light or heat Benzyl chloride industrial process highly regioselective for benzylic position Benzylic Bromination Free-radical Chlorination of Toluene Similarly, dichlorination and trichlorination are selective for the benzylic carbon. Further chlorination gives: is used in the laboratory to introduce a halogen at the benzylic position CH2Br CH3 CHCl2 (Dichloromethyl) benzene CCl3 (Dichloromethyl) benzene N-Bromosuccinimide (NBS) is a convenient reagent for benzylic bromination Br CH2CH3 O NBr + O CCl4 benzoyl peroxide, heat CHCH3 O NH + O (87%) + Br2 NO2 p-Nitrotoluene CCl4, 80°C + HBr light NO2 p-Nitrobenzyl bromide (71%) Site of Oxidation is Benzylic Carbon Example O CH3 or CH2R or CH3 Na2Cr2O7 H2SO4 O COH H 2O heat p-Nitrotoluene Example O CH(CH3)2 COH Na2Cr2O7 H2SO4 H 2O heat CH3 H 2O heat NO2 CHR2 COH O (45%) COH Na2Cr2O7 H2SO4 NO2 p-Nitrobenzoic acid (82-86%) Benzylic SN1 Reactions Benzylic SN1 Reactions Relative solvolysis rates in aqueous acetone: CH3 C CH3 Cl CH3 CH3 C CH3 Cl CH3 C+ CH3 CH3 CH3 620 Relative rates of formation: 1 C+ CH3 more stable less stable Tertiary benzylic carbocation is formed more rapidly than tertiary carbocation; therefore, more stable. Compare Resonance in Benzyl Cation H C C C+ allylic carbocation + C H H H H H C+ benzylic carbocation Benzylic carbon is analogous to allylic carbon. H Positive charge is delocalized between benzylic carbon and the ring carbons that are ortho and para to it. Resonance in Benzyl Cation H H Resonance in Benzyl Cation H C H H + H H H C H H + H H H H Positive charge is delocalized between benzylic carbon and the ring carbons that are ortho and para to it. Resonance in Benzyl Cation Positive charge is delocalized between benzylic carbon and the ring carbons that are ortho and para to it. Solvolysis CH3 H H C H + H C H H H Positive charge is delocalized between benzylic carbon and the ring carbons that are ortho and para to it. Cl CH3 CH3CH2OH CH3 C CH3 OCH2CH3 (87%) Primary Benzylic Halides O2N CH2Cl Mechanism is SN2 O Like allylic halide, substitution is faster than a normal primary halide. NaOCCH3 acetic acid O O2N CH2OCCH3 (78-82%) Dehydrogenation • Industrial preparation of styrene • Almost 12 billion lbs. produced annually in U.S. CH2CH3 630°C ZnO CH + H2 CH2 Dehydrohalogenation Acid-Catalyzed Dehydration of Benzylic Alcohols Cl Br KHSO4 CHCH3 CH2CHCH3 H 3C Cl CH heat CH2 NaOCH2CH3 (80-82%) OH ethanol, 50°C Cl CH H 3C CHCH3 + CHCH3 (99%) Hydrogenation CH3 Addition Reactions of Alkenylbenzenes C CH3 CHCH3 CHCH2CH3 H2 Pt Br Br (92%) Halogenation Addition of Hydrogen Halides Cl CH CH2 HCl Br2 CH CH2 Br Br (82%) (75-84%) + via benzylic carbocation Free-Radical Addition of HBr CH CH2 HBr peroxides CH2CH2Br Polymerization of Styrene CH • CH2Br via benzylic radical Polymerization of Styrene H 2C CH2 CH C 6H 5 C 6H 5 •• RO •• • CHC6H5 CH2 CH Mechanism CH2 H 2C CH CHC6H5 C 6H 5 polystyrene Mechanism •• RO •• H 2C Mechanism •• RO •• H 2C CHC6H5 • H 2C CHC6H5 CHC6H5 • H 2C CHC6H5 Mechanism •• RO •• H 2C •• RO •• H 2C CHC6H5 H 2C CHC6H5 Mechanism •• RO •• CHC6H5 H 2C CHC6H5 H 2C CHC6H5 H 2C CHC6H5 • H 2C H 2C Mechanism CHC6H5 • H 2C CHC6H5 CHC6H5 • H 2C CHC6H5 120 Heats of Hydrogenation Heats of Hydrogenation To give cyclohexane (kJ/mol): To give cyclooctane (kJ/mol): 231 208 Heat of hydrogenation of benzene is 152 kJ/mol, less than 3 times heat of hydrogenation of cyclohexene. Structure of Cyclooctatetraene Cyclooctatetraene is not planar; has alternating long (146 pm) and short (133 pm) bonds. 97 205 303 410 Heat of hydrogenation of cyclooctatetraene is more than 4 times heat of hydrogenation of cyclooctene. Structure of Cyclobutadiene MO calculations give alternating short and long bonds for cyclobutadiene. H H 135 pm 156 pm H H Stability of Cyclobutadiene Cyclobutadiene is observed to be highly reactive, and too unstable to be isolated and stored in the customary way. Requirements for Aromaticity Cyclic conjugation is necessary, but not sufficient. Not only is cyclobutadiene not aromatic, it is antiaromatic. An antiaromatic substance is one that is destabilized by cyclic conjugation. aromatic Antiaromatic when square. Conclusion There must be some factor in addition to cyclic conjugation that determines whether or not a molecule is aromatic. Antiaromatic when planar. Hückel's Rule Among planar, monocyclic, completely conjugated polyenes, only those with 4n + 2 π electrons possess special stability (are aromatic). n 4n+2 0 2 1 6 2 10 3 14 4 18 Benzene! Hückel's Rule Hückel restricted his analysis to planar, completely conjugated, monocyclic polyenes. He found that the π molecular orbitals of these compounds had a distinctive pattern. One π orbital was lowest in energy, another was highest in energy, and the others were arranged in pairs between the highest and the lowest. Frost's Circle Hückel's Rule Frost's circle is a mnemonic that allows us to draw a diagram showing the relative energies of the π orbitals of a cyclic conjugated system. 1) Draw a circle. 2) Inscribe a regular polygon inside the circle so that one of its corners is at the bottom. 3) Every point where a corner of the polygon touches the circle corresponds to a π electron energy level. 4) The middle of the circle separates bonding and antibonding orbitals. Antibonding Bonding π MOs of Benzene π-MOs of Benzene π-MOs of Benzene Antibonding Benzene Antibonding Benzene Bonding 6 p orbitals give 6 π orbitals. 3 orbitals are bonding; 3 are antibonding. Bonding 6 π electrons fill all of the bonding orbitals. All π antibonding orbitals are empty. π-MOs of Cyclobutadiene (Square Planar) π-MOs of Cyclobutadiene (Square Planar) Antibonding Cyclobutadiene 4 p orbitals give 4π orbitals. 1 orbital is bonding, one is antibonding, and 2 are nonbonding. Bonding Antibonding Cyclobutadiene Bonding 4 π electrons; bonding orbital is filled; other 2 π electrons singly occupy two nonbonding orbitals. π-MOs of Cyclooctatetraene (Planar) π-MOs of Cyclooctatetraene (Planar) Antibonding Cyclooctatetraene Bonding 8 p orbitals give 8 π orbitals. 3 orbitals are bonding, 3 are antibonding, and 2 are nonbonding. π-Electron Requirement for Aromaticity 4 π electrons 6 π electrons 8 π electrons not aromatic aromatic not aromatic Antibonding Cyclooctatetraene Bonding 8 π electrons; 3 bonding orbitals are filled; 2 nonbonding orbitals are each half-filled. Completely Conjugated Polyenes 6 π electrons; completely conjugated 6 π electrons; not completely conjugated H aromatic H not aromatic Annulenes Annulenes are planar, monocyclic, completely conjugated polyenes. That is, they are the kind of hydrocarbons treated by Hückel's rule. [10]Annulene [10]Annulene van der Waals strain between these two hydrogens Predicted to be aromatic by Hückel's rule, but too much angle strain when planar and all double bonds are cis. 10-sided regular polygon has angles of 144°. Incorporating two trans double bonds into the ring relieves angle strain but introduces van der Waals strain into the structure and causes the ring to be distorted from planarity. [14]Annulene [16]Annulene HH HH 14 π electrons satisfies Hückel's rule. 16 π electrons does not satisfy Hückel's rule. van der Waals strain between hydrogens inside the ring. Alternating short (134 pm) and long (146 pm) bonds. [18]Annulene H H H H H H 18 π electrons satisfies Hückel's rule. Resonance energy = 418 kJ/mol. Bond distances range between 137-143 pm. Is an antiaromatic 4n π-electron system. Cycloheptatrienyl Cation H H H H H + H H Cycloheptatrienyl Cation 6 π electrons delocalized over 7 carbons positive charge dispersed over 7 carbons very stable carbocation also called tropylium cation H H H H + H H H + H H H H Cyclopentadienide Anion H Br– H Ionic H H Cycloheptatrienyl Cation + H Br H H •• Covalent Tropylium cation is so stable that tropylium bromide is ionic rather than covalent. mp 203 °C; soluble in water; insoluble in diethyl ether H – H 6 π electrons delocalized over 5 carbons negative charge dispersed over 5 carbons stabilized anion Cyclopentadienide Anion Acidity of Cyclopentadiene H H H •• H H H – H H H H H •• – H H H H H H H H H H H H H H H H •• H •• H – H H H H H – H H H H – •• H H H H – Compare Acidities of Cyclopentadiene and Cycloheptatriene – •• H •• H Cyclopentadiene is unusually acidic for a hydrocarbon. Increased acidity is due to stability of cyclopentadienide anion. Ka = 10-16 H H H pKa = 16 Electron Delocalization in Cyclopentadienide Anion H+ + H H H H H H – H H H H H H H pKa = 16 pKa = 36 Ka = 10-16 Ka = 10-36 Cyclopropenyl Cation Compare Acidities of Cyclopentadiene and Cycloheptatriene H H H H H H also written as + H H •• – H H Aromatic anion 6 π electrons H H •• – H H H + H H n=0 4n +2 = 2 π electrons H Antiaromatic anion 8 π electrons Examples N •• •• N •• •• O S Furan Thiophene •• •• H Pyridine Pyrrole Examples N •• N •• Quinoline Isoquinoline Pyridine Pyrrole 6 π electrons in ring. Lone pair on nitrogen is in an N •• sp2 hybridized orbital; not part of π system of ring. Lone pair on nitrogen must be part •• N H of ring π system if ring is to have 6 π electrons. Lone pair must be in a p orbital in order to overlap with ring π system. Furan Two lone pairs on oxygen. One pair is in a p orbital and is part •• O •• of ring π system; other is in an sp2 hybridized orbital and is not part of ring π system.