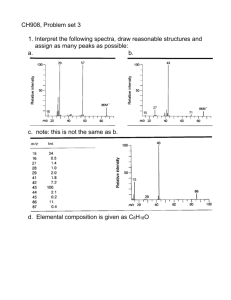
Mass Spectrometry and Free Radicals MS recap Positive mode of
advertisement

Mass Spectrometry and Free Radicals MS recap Positive mode of Electron Ionization / ESI MS sees charged species (M+•, M+), but does not see uncharged (R•) Radical and Cation Stabilities Radical vs cation stabilities 1 BDE, Reactions with H abstractors, fragmentation. Stabilities, and likelihood of a process occurring is reflected in the height of the MS peaks. 2 Mass Spectrometry and Free Radicals (really radical cations) When an organic molecule is ionized in an MS instrument, especially by EI , it generates a radical cation. M+• Often there is confusion or ambiguity over what a radical cation really looks like – meaning – which electron was removed, and where the + is and where the • is. 3 Sometimes we can definitely tell, sometimes we cannot, depending on how accurate our Lewis Structure description is. E.g. clear cut case is CH4 But cyclohexadiene is ambiguous (because Lewis Structure is imprecise). 4 The ionization process knocks out (EI) an electron. It makes sense that it is the electron which is easiest to remove. This can be one from a lone pair π bond σ bond (These are listed in decreasing ease – so a lone pair electron is the easiest to remove). 5 For MS, we really need to think about two things: - what will the M+• look like ? - How will it fragment (and maybe rearrange) ? Different M+• -Alkanes -Alkenes (and carbon-carbon pi bonds) -Heteroatoms (alcohols, amines, ethers, halogens) -Aldehydes and Ketones 6 For alkanes, the easiest electron to remove is going to be in a sigma bond. In ethane, potentially a C-H or C-C bond. But almost always the weakest bond is the C-C bond. (BDE ethane C-H = 98kcal/mol, C-C = 83kcal/mol) (The electron removed in decane is slightly harder to exactly predict, since there are many “similar energy” C-C choices). 7 For alkenes, one of the pi electrons will be the one most easily removed. So for ethene: Notice that for unsymmetrical alkenes (like propene), it starts to introduce some ambiguity as to which C has the +, and which has the • 8 For molecules that have heteroatoms like N, O etc, and also halogens, the lone pairs of electrons are the least tightly held (i.e. highest energy, easiest to remove), so one of the lone pair electrons will usually get removed. E.g. ethyl ether Or bromopropane. 9 For aldehydes and ketones, one of the Oxygen lone pair electrons gets removed. So for acetone, and for ethanal. . 10 In most cases, the radical cation will fragment. This is what generates the other peaks in the mass spectrum. This fragmentation is just free radical chemistry, like we have been previously studying. The only difference is we are starting from a radical cation. But it is the radical character that dictates the reactivity and chemistry. (You may view the MS process as a radical process, but instead of using a typical free radical initiator like AIBN or peroxides, we use the ionization technique to generate our reactive species, which is a radical cation). The fact that this “chemistry” is occurring in a vacuum, means that there are no bimolecular reactions (like additions, dimerizations, etc), but only unimolecular processes. We observe “what species do when there is nothing else for it to react with”. Fragmentation processes are always entropically favourable (1 goes to 2 species) and can also be enthalpically favourable also since radical cations are usually unstable. If the radical cation IS relatively stable, then it will not do anything (and therefore give a dominant M+• peak in the MS). 11 Fragmentation of Alkanes The radical cation of an alkane will fragment to yield a radical and a cation. (And also we must consider the likelihood of it fragmenting “the other way”). E.g. The electron removed, and the consequential bond breaking are not random, but are controlled by the desire to form the most stable fragments. Often in long chain alkanes, this leads to many fragmentation processes. 12 For the case of n-hexane: The weakest bonds are: the C-C bond between C2 and C3, and the C-C bond between C3 and C4. These are the two most likely locations for ionization (other are less likely – not impossible – just lower probability = smaller peaks in MS). (When comparing two straight chains, typically the longer chain prefers the cation vs the radical.) For the radical cation located at C3-C4, fragmentation leads to a propyl radical (MS invisible) and a 1-propyl cation (m/z = 43). (1-Propyl means + on C1) (Symmetrical so only 1 possible fragmentation). 13 For the radical cation located at C2-C3, this could fragment to yield either a: - 1-Butyl cation and ethyl radical, or 1-Butyl radical and ethyl cation Both of these processes are energetically reasonable, and therefore both processes occur. (When comparing two straight chains, typically the longer chain prefers the cation vs the radical.) 14 The chances of having the radical cation located at C1-C2 is low since cleavage of that species would generate either a: - 1-Pentyl cation and methyl radical - 1-Pentyl radical and methyl cation The methyl radical is very unstable (so any process that generates it is undesirable), and the methyl cation is even more unstable, and will not form). 15 All of these competing process are reflected in the MS of hexane. MS spectrum reflects the (i) statistics (twice as many C2-C3 bonds), and (ii) relative stabilities. 16 With branched alkanes, fragmentation commonly occurs at a branch carbon atom, since this will generate the most highly substituted radicals and cations (and hence the most stable radicals and cations). For example, when 2-methyl pentane is ionized, the most likely locations of the radical cation are C1-C2 and C2-C3. For C1-C2, the fragmentation could yield - 2-pentyl cation and methyl radical (better than 2-pentyl radical / methyl cation) C2-C3 fragmentation could yield - 2-propyl cation and 1-propyl radical (better than 2-propyl radical and 1-propyl cation) 17 An assessment of the stabilities of BOTH the radical AND cation species formed for each process leads us to predict the 2nd one to be the major process. As expected there is a dominant m/z 43 peak in the MS. 18 In the case of alkenes, where a π electron has been removed, the most common type of fragmentation involves the fragmentation of an allylic bond, to generate a RESONANCE stabilized allylic cation. e.g. 19 For 2-hexene, the radical cation can be written thus, and if we cleave C4-C5, we generate a radical fragment and a species with a new π bond and carbocation. So the MS of 2-hexene shows a big peak at m/z=55. 20 Relatedly, compounds containing an alkyl substituted aromatic ring tend to fragment between the alpha-beta carbons from the ring, as this generates the benzylic carbocation (which may further rearrange to the tropylium ion). 21 Often, aromatic compounds that do not have fragmentable substituents, or only H, just show the M+• peak. (i.e. they don’t fragment). E.g. Naphthalene 22 For ethers, one of the lone pair electrons gets removed and then there is either - cleavage of the alpha – beta bond (yielding a resonance stabilized cation) - C-O heterolytic cleavage generating an alkoxyl radical, and hydrocarbon carbocation. 23 For amines, one of the lone pair electrons gets removed and then there is - cleavage of the alpha – beta bond (yielding a resonance stablized cation) - The R’ fragment lost is usually the one which can best support a radical (i.e. most branching, or longest straight chain). 24 For alcohols, one of the lone pair electrons gets removed and then there is either - cleavage of the hydroxyl carbon – alpha carbon bond (yielding a resonance stabilized cation). - Dehydration yielding an alkene radical cation. 25 The MS spectrum of 3-methyl-1-butanol (MW=88) reveals a strong... (M-18) peak (dehydration) and (M-33) peak (cleavage) 26 For carbonyl compounds such as aldehydes and ketones, one of the oxygen lone pair electrons is removed, which is usually followed by cleavage of the carbonyl C-alpha C bond, which results in a resonance stabilized acylium ion and substituent fragment. Simple straight chain ketones will often fragment in “both directions” 27 Simple straight chain ketones will often fragment in “both directions” e.g. MS of 2-butanone. 28 29 Rearrangements / Other These occur IN ADDITION to the regular MS fragmentation processes. (1) McLafferty Rearrangement (2) Cyclizations (1) The McLafferty Rearrangement occurs with compounds that have a C=O, and an available gamma hydrogen. It occurs for any C=O including aldehydes, ketones, esters, acids, etc with a 3 or more carbon chain substituent. 30 In a 6 membered transition state, there is H abstraction by the O, formation of a new pi bond, and cleavage of the bond alpha and beta to the carbonyl carbon. It generates a neutral alkene (MS invisible) and an enol radical cation. 31 It even occurs in appropriate straight chain nitriles. 32 (2) Cyclizations For longer chain amines and primary amides, there can be fragmentation leading to cyclization. Usually this leads to 5 or 6 membered ring products (cations) and an alkyl radical. E.g. hexanamine 33 This also happens for Primary amides. 34