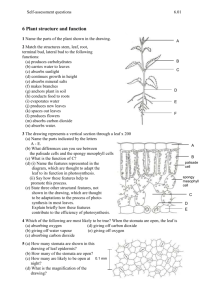
Primary Producers
advertisement

© Dr. Bibit Traut Botany 10 LAB Spring 2011 Osmosis & Photosynthesis I. Osmosis 1) Osmosis is the diffusion of water through a semi-permeable membrane. This process is driven by the movement of water (the solvent) from a region where water is more concentrated to region where it is less concentrated. In other words, water will move to from regions of low solute concentration to regions of high solute concentration. The process of osmosis is important in many plant processes, especially translocation and transpiration. 2) Osmosis concentration experiment a) Dialysis tubing will serve as the differentially-permeable membrane in this simulation of artificial cells, allowing only the water to diffuse through it. You will observe the effect of cells with differing solute concentration to illustrate the effect of solute concentration on osmosis. b) Procedure: i) Cut three 12.5 cm pieces of dialysis tubing and soak them in DI water. ii) Close one end of each dialysis tubing by tying it closed with string. iii) Fill each tube with one of each of 3 different solutions (1) 15 ml of sugar syrup solution (1:2 dilution) (2) 15 ml of sugar syrup solution (1:4 dilution) (3) 15 ml of distilled water iv) Make sure dialysis tubing with its solution is not tight, but flaccid, then tie off the other end to seal and rinse off with DI water (be sure string is wet too. Why?). Dry bag and weigh to the nearest gram. v) Place each bag in a separate 600 ml beaker with 400 ml of distilled water in it. Make sure the beaker is labeled with the concentration of the solute in the dialysis tubing. vi) Weigh bags in grams (dried bag) initially and then at 10 minutes, 20 minutes, and 30 minutes. c) Does beaker water change color? Same for each bag? What had the greatest rate of osmosis? How might plants use this osmotic gradient as a “pump”? II. Guard cell response to osmotic pressure 1) The opening of guard cells is achieved by turgor pressure in these crescent shaped cells. When water is readily available, the guard cells will take in water, causing them to expand differentially due to cellulose fibers that run through them. When plants are water stressed, a process initiates that changes the osmotic potential inside of the guard cells and water leaves them, causing them to deflate and shut. You will observe this process by simulating a change in water potential by adding a salt solution (5% NaCl) to epidermal tissue. 2) Procedure: a) Obtain a leaf specimen from your instructor (e.g. Zebrinia). Bend it in half with the underside inward in order to cause the whole leaf to crack. Next use forceps to peal off the thin epidermal tissue and mount on a slide in a drop of distilled water and cover with a coverslip. Observe and sketch the stomata. -1- © Dr. Bibit Traut Botany 10 LAB Spring 2011 b) What do you think will happen to the stomata if you add a salt solution to the epidermis? Why? Add 5% NaCl to the specimen by drawing it through under the coverslip (by applying a drop on one side of the coverslip and having a kimwipe on the opposite edge of the coverslilp). Observe and sketch the stomata. c) Questions/observations: i) Why do you think stomata are open when water is plentiful? ii) How do the stomata respond to changes in water potential/turgor pressur? What mechanisms are involved? iii) Why were you able to use a salt solution to mimic changes in water availablity in a leaf that effects the stomata opening? III. Photosynthesis 1) Relationship of Photosynthesis and CO2 a) Recall the relationship between photosynthesis and the utlilization of CO2. For this experiment you will observe this relationship. b) Procedure: i) Fill a test tube with water and then add a dropperful of phenol red, so that the solution turns pink/red. Phenol red is a pH indicator that turns red when pH is 7 or greater and yellow when pH is less than 7. Because of the bicarbonate/carbonic reaction, when CO2 decreases the solution will be more basic and the solution will be red, however when CO2 increases the solution becomes more acidic and yellow. ii) With a straw, blow into the tube until the solution turns yellow (acidic). What are you adding to the solution by doing this? iii) Empty half of this solution into another test tube and add a sprig of Elodea. iv) Place both test tubes in bright light for ~20 minutes. How do the two tubes differ after this time? Why did the color change in one tube and not in the other? 2) Paper Chromotagraphy a) Paper chromatography is a technique that is used to separate plant pigments. This process works by using the differences in charges of molecules and differences in polarity of chromatography paper and non-polarity of petroleum ether solvent. The pigments are more or less polar (see handout) and because of this can be separated based on their differential attraction or lack of attraction to the chromatography paper. Remember, that like attracts like (polar binds to polar, nonpolar binds to nonpolar). b) Procedure i) You have pre-cut strip of chromatography paper. DO NOT TOUCH!! Use your forceps to handle the paper. Fit the chromatography paper in your corked tube so that it hangs about half a centimeter above the bottom. You will add solvent to the bottom of the tube so that it will contact the paper, but be below a pigment spot that you have made on the paper. ii) Remove the paper and place pigment extract on the bottom of the paper using the capillary tube to repeatably apply and make a dark spot (about 1cm up, so that when the paper hangs in the tube, the paper will contact the small amount of solvent in the bottom of the tube, but the pigment drop is above it). -2- © Dr. Bibit Traut Botany 10 LAB Spring 2011 iii) Hang the paper in the tube and allow the solvent, being careful that when you place the paper in, it does not go too deep and the pigment spot gets “washed” out into the solvent. Next, the solvent will begin to move up the paper, “grabbing” pigments as it goes and separating the 4 major photosynthetic pigments. Remove the paper from the tube once the solvent reaches the paperclip from which the paper is hanging. c) Describe how the pigments separated and identify each pigment on your paper. 3) Relationship of Photosynthesis to Chlorophyll and Starch Accumulation a) Photosynthetically active cells necessarily contain chlorophyll. The product of photosynthesis accumulates and is stored as starch. Some plants are variegated and have areas lacking pigment and therefore appear white or pink/red in parts and green in other areas (with chlorophyll-containing cells). Coleus is an example of such a plant. In this lab, you will utilize this difference to observe the relationship between photosynthesis, chlorophyll and starch. b) Procedure: i) Obtain both a masked (covered for 5 days in foil) and unmasked Coleus leaf. Sketch an outline of the leaf and note where the green parts are. You will repeat the process below for each leaf and then compare the amount and distribution of starch in each of them. ii) Using tongs, immerse the leaf (either the masked or unmasked—KEEP TRACK) in boiling water for ~10-15 seconds to break down the plasma membranes of the cells. Then transfer the leaf to boiling ethanol to extract the chlorophyll from the leaf. When the leaf has lost most of its pigments, remove it and rinse it with water. iii) Place the leaf in a Petri dish and add iodine dye so that the surface is covered and the starch is stained. Describe the results, focusing on how the masked leaf differed from the unmasked leaf and what causes this. IV. Questions for Review 1. How does an osmotic pump work to move sugars throughout a plant? 2. Assume that the Elodea sprig in the phenol red solution carries on photosynthesis. Does the oxygen given off have anything to do with the color change? 3. What serves as evidence that chlorophyll has been removed from the Coleus leaf? 4. During paper chromatography, what pigments were golden-yellow? What light would be absorbed by these pigments? 5. How is osmosis involved in the opening of guard cells? How does water stress or wilting of a plant affect the opening/closing of stomata? -3-