Diffraction Grating Spectrometer Lab Report
advertisement

2B30: PRACTICAL ASTROPHYSICS
FORMAL REPORT:
O6: The Diffraction
Grating Spectrometer
Adam Hill
Lab partner: G. Evans
Tutor: Dr. Peter Storey
1
Abstract
The calibration of a diffraction grating spectrometer using a source of known wavelength
was made. The calibrated spectrometer was then used to determine the wavelengths of
lines in the spectra of atomic hydrogen and other atoms. This was achieved by
calibrating a transmission diffraction grating using a sodium spectral source, whose line
wavelengths were well known. The spectral lines in atomic hydrogen and helium were
then measured and their corresponding wavelengths calculated using the data obtained
through the calibration. The experimental results yielded an approximation of the
Rydberg constant, R, was found; R ≈ 1.09 x 10-7 m-1.
2
Contents
Abstract
2
Contents
3
Introduction
4
Experimental Method
5
Experimental Data
• Diffraction Grating Calibration
• Measuring the Sodium doublet
• Measuring the Hydrogen Balmer series
• Measuring the Helium spectral lines
7
8
9
10
Error Analysis
11
Summary of Results
11
Conclusion
13
Appendix A
14
References
15
3
Introduction
When atoms or molecules are excited, for example through heating, then electrons gain
energy and are promoted to higher energy levels within the atom or molecule. When the
electrons drop down again a photon of light is given off with a specific wavelength
corresponding to the energy lost by the electron. Every element or compound has a
specific line spectrum associated with it. This leads to spectroscopy being a fundamental
tool used by astronomers to determine what astronomical bodies are made up of.
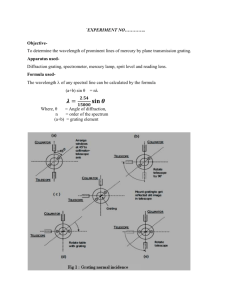
In this experiment a transmission diffraction grating is used. This consists of a mask with
a large number of evenly spaced slits. A light from the source passes through a
collimator so that parallel rays of light fall onto the diffraction grating. The rays of light
are diffracted through the slits and the diffracted rays recombined to form an image of the
collimator slit at the telescope focus. For the diffracted image to have a maximum
intensity the path difference between adjacent slits must contain an integral number of
wavelengths, see Fig. 1.
Figure 1: Diffraction at the grating
θi
θp
This results in the equation,
1/N (sinθp - sinθi) = pλ
Where: θp = diffracted angle
θi = incident angle
N = number of lines per unit length of grating
p = order of interference
λ = wavelength of light
4
(1)
1/N
The angular deviation of the beam, D, is given by the formula below,
D = θp - θi
(2)
The undeviated beam, when D=0, corresponds to the zeroth order, i.e. p=0.
When the grating is normal to the beam, θi =0°, θp=D. From equation (1) we thus get,
SinD = pNλ
(3)
Experimental Method
The experimental apparatus was set up as shown below, Fig. 2.
Figure 2: Experimental apparatus
Diffraction
grating
Turntable
Spectra
source
slit
Telescope
vernier
Collimator
Turntable
vernier
Telescope
5
Angular scale
Procedure
Before any measurements were taken the spectrometer had to be adjusted and calibrated.
•
•
•
The telescope eyepiece was focussed on the crosswire and adjusted until the
crosswire was in sharp focus.
The axes of the telescope and collimator were set to be perpendicular.
The vertical angle of the grating plane was made perpendicular to the incident
beam and the grating is set to be normal to the beam in the horizontal plane.
To make these adjustments the following procedure was carried out.
1. The telescope was set on the undeviated image of the slit and its angular
position recorded.
2. The telescope was rotated through ninety degrees to make the telescope
and collimator axes perpendicular.
3. The grating was rotated so that the reflected image from its front was
centred in the telescope. This set the grating at a 45° angle to the
horizontal.
4. To adjust the vertical angle of the grating, the vertical centre of the
collimator slit was reduced in length to a small element about the centre.
5. The reflected image was observed in the telescope and the grating
levelling screws adjusted until the slit centre was aligned with the
horizontal crosswire.
6. The grating angle was recorded and the grating rotated 45° to make it
perpendicular to the collimator axis.
•
•
To ensure that the grating rulings were parallel to the spectrometer axis, the
levelling screws were adjusted such that on rotation of the telescope the image of
the slit centre stays in the same place.
The collimator slit was made parallel to the grating rulings.
6
Experimental Data
Calibration of the grating
Through the calibration of the grating we found the number of lines per unit length of
the grating. This was done using the sodium lamp as the light source. The diffraction
angle, θp (deviation from straight through image), was measured for one of the
sodium lines in as many orders as possible.
Table 1
Telescope Angle
θp
Sin (θ
θp)
(º)
(º)
(4 s.f.)
0
220.1011
0.0000
0
1
210.0333
10.0678
0.1748
2
199.5000
20.6011
0.3519
3
188.0056
32.0956
0.5313
4
174.5611
45.5400
0.7137
5
155.6389
64.4622
0.9023
-1
230.1861
-10.0850
-0.1751
-2
240.4278
-20.3267
-0.3474
-3
251.2750
-31.1739
-0.5176
-4
262.2389
-42.1378
-0.6709
Order (p)
The data in Table 1 was plotted resulting in Graph 1.
To find the equation of the line the least squares method was used. This yielded a
measurement for the gradient of the line, m, of m = 1.75 +/- 0.01 x10-1.
Equation (3) gave,
Sin(D) = N p
Therefore the gradient of the line should be m = N .
N = 1.75 +/- 0.01 x10-1
= 589 x 10-9 m
N = 297 +/- 2 x 103 m-1
This gives the number of lines per mm for the diffraction grating at,
N ≈ 300 lines mm-1
(2 s.f.)
This is an appropriate result for a diffraction grating.
7
The Sodium Doublet
The separation of the sodium doublet was also measured to as many orders as
possible. The data collected is displayed below in Table 2.
Table 2
Order
(p)
0
1
2
3
4
-1
-2
-3
Telescope
Angle 1
(º)
220.106
209.869
198.972
186.750
171.603
230.153
240.408
250.769
D1
(º)
0.000
10.236
21.133
33.356
48.503
-10.047
-20.303
-30.664
Telescope
Angle 2
(º)
220.106
209.861
198.919
186.681
171.525
230.178
240.422
250.792
D2
(º)
0.000
10.244
21.186
33.425
48.581
-10.072
-20.317
-30.686
Mod
Cos(D) ∆λ ((nm)
(D2 - D1)
(4 s.f.) (4 s.f.)
0.008 0.9841 0.4771
0.053 0.9327 1.4320
0.069 0.8353 1.1249
0.078 0.6626 0.7495
0.025 0.9847 1.4321
0.014 0.9379 0.3789
0.022 0.8602 0.3707
The data shown in Table 2 was used to calculate the angular separation of the sodium
doublet.
Equation (3) gives,
Sin(D) = N p
Differentiating gives,
Cos(D) ∆D = pN∆λ
This gives,
∆λ = Cos(D) ∆D/ pN
(4)
Using the data in table 2 and the statistical equations from Appendix A, the mean,
standard deviation, standard deviation of the sample and standard error on the mean
can be found.
< ∆λ > = 8.809 x 10-10 m
s
= 4.226 x 10-10 m
σ
= 4.564 x 10-10 m
σm
= 1.597 x 10-10 m
(4 s.f.)
(4 s.f.)
(4 s.f.)
(4 s.f.)
This gives an average value for ∆λ as,
0.8 +/- 0.2 nm
(4 s.f.)
The sodium doublet is found at 589nm and 589.6 nm. This gives a true value of ∆λ of
0.6 nm. This is within the uncertainty of the experimental measurement.
8
Measurement of the Balmer Series
With the diffraction grating calibrated it became possible to measure the Hydrogen
Balmer series. The angle of the violet, green and red lines produced by the hydrogen
source were measured to as high an order as possible, the results are displayed in Table 3
below.
Table 3
Order
0
1
2
3
4
5
-1
-2
Violet line
220.1083
213.9
205.2944
197.9
187.6694
181.3056
227.7056
234.7472
Telescope Angle (º)
Green/blue line
220.1083
211.9667
203.6167
195
185.8667
175.7361
229.0361
237.4167
Red line
220.1083
209.0861
197.6556
185.4083
171.1444
151.8667
231.6583
243.9194
The telescope angles found in Table 3 together with equation (3) can be used to calculate
the wavelengths of the lines.
Table 4
Order
1
2
3
4
5
-1
-2
Violet line
DV
Sin (DV)
6.2083 0.1081
14.8139 0.2557
22.2083 0.3780
32.4389 0.5364
38.8028 0.6266
-7.5972 -0.1322
-14.6389 -0.2527
Green/blue line
DG
Sin (DG)
8.1417 0.1416
16.4917 0.2839
25.1083 0.4243
34.2417 0.5627
44.3722 0.6993
-8.9278 -0.1552
-17.3083 -0.2975
Red line
DR
Sin (DR)
11.0222 0.1912
22.4528 0.3819
34.7000 0.5693
48.9639 0.7543
68.2417 0.9288
-11.5500 -0.2002
-23.8111 -0.4037
The data in Table 4 is plotted in Graph 2. To find the equations of the lines of best fit
that would correspond to the plots in Graph 2, the least squares method was applied. This
gives us the gradient of each line.
Using equation (3) we find that the gradient of each line should be Nλ.
Violet line,
m = 1.28 +/- 0.02 x10-1
λ = 431.1 nm
(4 s.f.)
Green line,
m = 1.426 +/- 0.009 x10-1
λ = 479.3 nm
(4 s.f.)
Red line,
m = 1.90 +/- 0.01 x10-1
λ = 640.1 nm
(4 s.f.)
9
Measurement of the atomic spectrum of Helium
The previous part of the experiment was repeated using a Helium source instead of the
Hydrogen lamp. The readings obtained are n Table 5 below.
Table 5
0rder
0
1
2
3
4
-1
-2
Telescope Angle (°°)
Violet
Green Orange
Red
220.1056 220.1056 220.1056 220.1056
212.5056 211.6333 209.8861 208.0611
204.75 203.8528 199.8694 197.1139
146.8722 194.1056 189.4 185.1472
189 184.8528 178.0917 171.1972
227.6722 228.8083 231.6722 232.2
235.7472 237.8528 241.325 244.3028
The telescope angles found in Table 5 together with equation (3) can be used to calculate
the wavelengths of the lines.
Table 6
Order
1
2
3
4
-1
-2
Violet line
DV
Sin (DV)
7.6000 0.1323
15.3556 0.2648
73.2333 0.9575
31.1056 0.5166
-7.5667 -0.1317
-15.6417 -0.2696
Green/blue line
DG
Sin (DG)
8.4722 0.1473
16.2528 0.2799
26.0000 0.4384
35.2528 0.5772
-8.7028 -0.1513
-17.7472 -0.3048
Orange line
DO
Sin (DO)
10.2194 0.1774
20.2361 0.3459
30.7056 0.5106
42.0139 0.6693
-11.5667 -0.2005
-21.2194 -0.3619
Red line
DR
Sin (DR)
12.0444 0.2087
22.9917 0.3906
34.9583 0.5730
48.9083 0.7537
-12.0944 -0.2095
-24.1972 -0.4099
Using the same method as for the Hydrogen Balmer series the wavelengths of the lines
can be calculated.
Violet line,
m = 1.312 +/- 0.009 x10-1
λ = 441.1 nm
(4 s.f.)
Green line,
m = 1.46 +/- 0.01 x10-1
λ = 492.6 nm
(4 s.f.)
Orange line,
m = 1.73 +/- 0.03 x10-1
λ = 583.3 nm
(4 s.f.)
Red line,
m = 1.94 +/- 0.03 x10-1
λ = 653.6 nm
(4 s.f.)
10
Error Analysis
The primary random error associated with this experiment is the human reading of the
rotation angle of the telescope. As an initial reading and then a final reading are taken
this error is compounded.
The error in the telescope scale is +/- 5”.
However due to human error in reading the Vernier scale a more realistic angle error
would be +/- 30”.
The overall error in D is,
(∆D)2 = (∆D1)2 + (∆D2)2
∴ ∆D = +/- 42”
This would affect the wavelength calculations as,
λ = SinD /pN
∴ ∆λ = cosD x ∆D/Np
∴ ∆λ/λ = +/- cot D x ∆D
This formula gives the error in each separate measurement of the wavelength of the
spectral lines. However the measurements obtained used least squares to find the overall
average wavelength making this formula difficult to apply to the collected data.
Summary of Results
The calibration of the diffraction grating yielded a value for N, the number of lines per
unit length of;
N ≈ 298 +/- 2 lines mm-1
The separation of the sodium doublet yielded a value of,
∆λ = 0.8 +/- 0.2 nm
The true value falls within the experimental value as, ∆λ =0.6 nm.
The wavelengths of the Hydrogen Balmer lines were found to be,
Colour
Measured value (nm) Uncertainty (nm) True Value (nm)1
Violet
431
7
434
Green/blue
479
3
486
Red
640
6
656
It can be seen that the true and measured values are similar but fall outside of the error.
11
The Rydberg constant, R is given by,
1/λij = RZ2 [1/j2 – 1/i2]
where,
Z = atomic number
j and i = energy levels within the atom.
For the violet line i=5; j=2
For the green line i=4; j=2
For the red line i=3; j=2
The corresponding approximations for R are:
Violet, R = 1.105 x 10-7 m-1
Green, R = 1.033 x 10-7 m-1
Red, R = 1.125 x 10-7 m-1
The mean value of R,
<R> = 1.09 x 10-7 m-1
The actual value of R is, R = 1.097 x 10-7 m-1 (2)
The wavelengths of the Helium spectrum were found to be,
Colour
Measured value (nm) Uncertainty (nm) True Value (nm)3
Violet
441
3
443.7
Green/blue
493
4
492
Orange
583
8
587
Red
654
9
656
It can be seen that the true values of the Helium spectral lines fall within the errors of the
experimental measurements.
12
Conclusion
The results obtained from the experiment gave approximate values of the separation of
the sodium doublet as,
0.8 +/- 0.2 nm
(1 s.f.)
The known separation of the doublet is 0.6 nm, which just falls within the experimental
uncertainty giving us an accurate result.
The values of the wavelengths of the Hydrogen Balmer lines were found to be,
Balmer line Measured value (nm) Uncertainty (nm) True Value (nm)1
431
7
434
χ
479
3
486
β
640
6
656
α
These values fall relatively close to the true values although they fall outside of the
errors.
The values for the Hydrogen Balmer series also gave rise to an approximation of the
Rydberg constant, R
R = 1.09 x 107 m-1
(3 s.f.)
The actual value of R is given as, R = 1.097 x 107 m-1 so the experimental measurement
was an accurate approximation.
The values of the Helium spectral lines were found to be,
Colour
Measured value (nm) Uncertainty (nm) True Value (nm)3
Violet
441
3
443.7
Green/blue
493
4
492
Orange
583
8
587
Red
654
9
656
The true values agree very closely with those measured and fall within the experimental
uncertainty.
The errors quoted in the results are statistical errors calculated from the sample data.
More precise analysis would include a method of calculating the known actual errors.
These could have been found for individual measurements but could not be incorporated
into the least squares method.
As the angular separation between lines is smaller at low orders any error in measurement
would have a larger effect on the calculations than the higher order measurements. This
may have been a large factor in the error on the sodium doublet. Low intensity lines at
higher orders for the Hydrogen and Helium spectra also made measurement difficult.
The precision of the experiment could be improved by using higher intensity sources,
more precise equipment – i.e. more accurate turntable mechanism, more measurements.
13
Appendix A
Statistical formulae (4)
The mean,
<x> = Σxi/N
The standard deviation,
s = √{[Σ(∆xi - <x>)2]/N}
The standard deviation,
of the sample.
σ = s√[N/N-1]
The standard error on the mean,
σm = s√[1/N]
14
References
1
The American Institute of Physics Handbook (12th edition)
2
Introduction to the Structure of Matter, JJ Brehm & WJ Mullin, Wiley
(inside cover)
3
The American Institute of Physics Handbook (12th edition)
4
Laboratory II: Student Handbook, session 1999/2000
(Data Analysis notes, Dr.MJ Esten)
15