TABLE II – Diffraction Angle Uncertainties, Wavelengths, and
advertisement
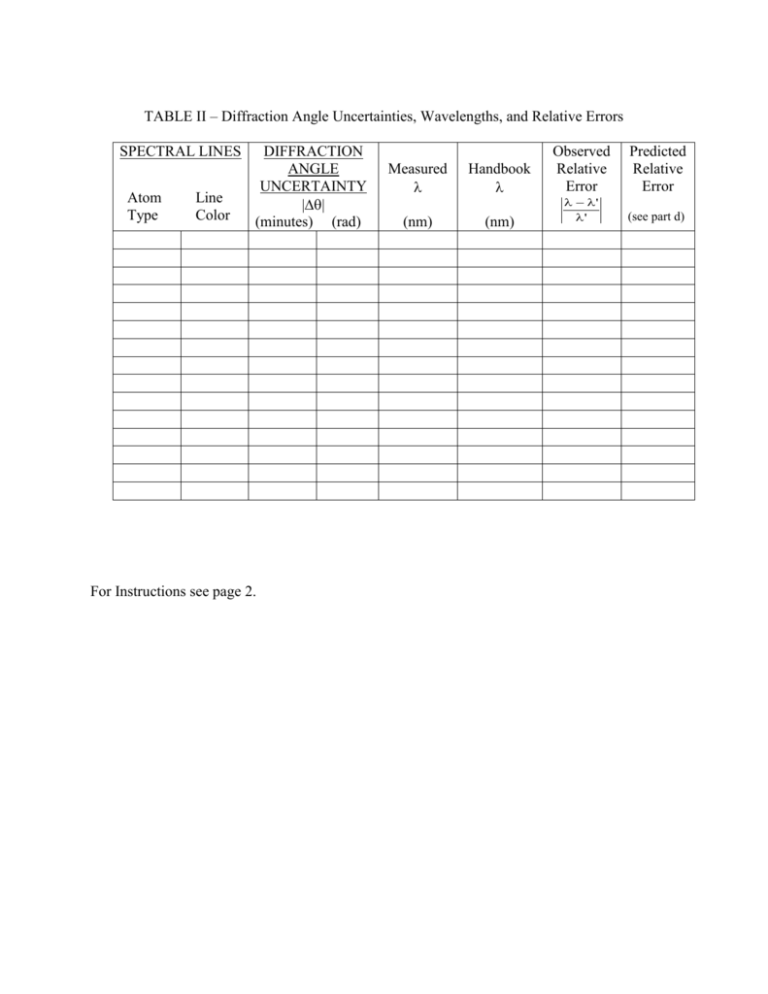
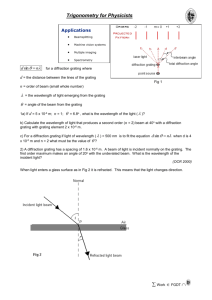
TABLE II – Diffraction Angle Uncertainties, Wavelengths, and Relative Errors SPECTRAL LINES Atom Type Line Color DIFFRACTION ANGLE UNCERTAINTY || (minutes) (rad) For Instructions see page 2. Measured Handbook (nm) (nm) Observed Relative Error Predicted Relative Error ' ' (see part d) a) To obtain the “Diffraction Angle Uncertainty”, do the following. For each Hydrogen line, write down the magnitude of the DIFFERENCE between (left) and (ave). This gives you a measure of the amount by which the angular readings for Hydrogen are uncertain. Pick the LARGEST of these differences and use it as the uncertainty for ALL the Hydrogen lines. (If all differences are zero, use 1 minute of arc as a default value for the smallest difference.) Report this both in units of minutes and of radians. Similarly, obtain a value of appropriate for the Mercury and Mystery measurements of the second lab period (one value of for the full set of measurements for Part II). b) “Measured ” values are calculated from the grating equation using (ave) as the diffraction angle. “Handbook ” values are obtained from page 4 (last week’s instructions). Because the mystery lamp wavelengths are not given, you will have to leave that entry for “Handbook ” blank. c) “Observed relative error” is defined as: ' ' d) “Predicted Relative Error” is determined from uncertainties present in this experiment. The calculated wavelength depends only on the diffraction angle and the grating spacing. By differentiating the grating equation, we find that the relative uncertainty in wavelength depends on the uncertainties in angle and grating spacing as follows: s s cos sin “s” is the grating constant given on your grating, “s” for these gratings is 0.3 lines/mm. “” is (ave) reported in Table I, and “” is the diffraction angle uncertainty – MEASURED IN RADIANS – reported in another column to the left. Make certain that you can see how this calculation works. When involved in measurements in other labs or eventually in your life’s work, you will be asked to specify uncertainties in measurements and related calculations. This is how it’s done.