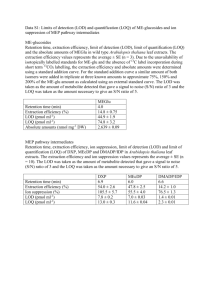
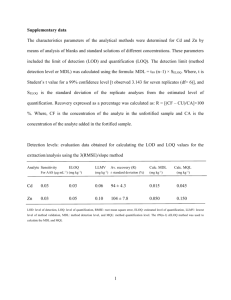
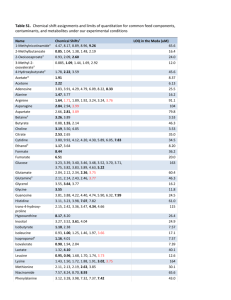
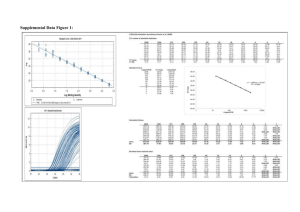
Determination of the Limit of Detection and the Limit of
advertisement

Determination of the Limit of Detection and the Limit of Quantitation during Assay Development Eloi P. Kpamegan, Ph.D., MSF Director, Clinical and Nonclinical Biostatistics Introduction ¾ The Limit of detection (LOD) and the limit of quantitation (LOQ) are important parameters that need to be determined during assay development. ¾ The usefulness and optimal throughput of an assay may depend on the appropriate determination of the LOD and the LOQ ¾ The experiment design and statistical method used for the determination of LOD and LOQ is dependent on the assay type (e.g., ELISA, Functional or PCR) ¾ This presentation describes the design, testing and statistical procedures required to determine the LOD and LOQ during assay development. The procedures to be used to confirm the LOD and the LOQ during assay validation are discussed Definition “Validation of Analytical Procedures is the process of determining the suitability of a given methodology for providing useful analytical data. A method that is valid in one situation could well be invalid in another.” J. Guerra, FDA, Pharm. Technology, March 1999 Definition Validation of an analytical method is primarily concerned with: • the identification of the sources of potential errors • quantification of the potential errors in the method An Assay Validation describes in mathematical and quantifiable terms the performance characteristics of an assay Common Misconceptions Assay Validation ≠ Assay Optimization ≠ Assay Qualification A Validated Method is NOT necessarily a “tight” method Repeating an assay a number of time does not constitute validation Evolvement of an Assay Development Optimization Qualification Pre-Validation Revalidation Validation Implementation Assay Validation Parameters: USP & ICH Accuracy Precision Limit of Detection Method Validation Limit of Quantitation Specificity Linearity and Range Ruggedness/Robustness System Suitability Assay Qualification ¾ An assay is qualified when the following parameters are assessed and the performance documented in a qualification (or Prevalidation) report LOB/LOD/LOQ Limited Precision/Reproducibility Accuracy Dilutability or Internal Accuracy Specificity Robustness Short-Term Stability Two Assay Types ¾Continuous Assays ELISA RIA Total IgG assay Etc ¾Functional assays Neutralization Assay PRNT Assay HAI Assay Etc Continuous Assays ¾Limit of Blank (LOB) – The LOB is the highest measurement result that is likely to be observed (with a stated probability) for a blank or negative sample ¾Limit of detection (LOD) – The LOD is the lowest amount of analyte in a sample that can be detected with (stated) probability, although not quantified as an exact value. Also called minimum detectable concentration (MDC) – The reliable detection limit (RDL) is an estimate of LOD that has a high probability of producing a response significantly greater than the response at zero concentration of analyte Æ When using a four parameter logistic reference curve the MDC and the RDL are two different estimates of the LOD ¾Limit of Quantitation (LOQ) – The LOQ is the lowest amount of analyte in a sample that can be quantitatively determined with a stated acceptable precision and accuracy, under stated experimental conditions Determination of LOB ¾The assumption in the determination of the LOB is that values exceeding the 95th percentile of the distribution of values on truly blank or negative samples deviate significantly from blank or negative measurements. When a sample produces an observed value that exceeds this limit, it may be declared to contain an amount of analyte that exceeds zero Æ NCCLS document EP17-A Determination of LOB Cont’ • A α value of 5% corresponds to using the 95th percentile of the distribution of blank or negative values as the limit for declaring a measured value significantly higher than the blank or negative • Given a Gaussian distribution of blank or negative values, this limit corresponds to: LOB = μ B + 1.645σ B where μB and σB are the mean and standard deviation of the blank or negative measurements, respectively LOD Experiment Design ¾ A minimum of 10 individual samples with concentrations ranging from the LOB to approximately 4xLOB shall be tested at least 10 times by at least two technicians in a minimum of two days or runs ¾ A pooled SDS estimate can be derived from repeated measurements with the set of samples (a minimum of 10 measurements of at least 10 samples) Measurements shall be carried out by different technicians in different days or runs to be able to capture the true variability of the analytical method. ¾ The SDS is the pooled estimate after checking the statistical assumption of homogeneity of variance LOD Estimation Cont’ An estimate LOD is then obtained as: LOD = LOB + cβSDS ¾ SDS is the estimated standard deviation of the sample distribution at a low level. ¾ As an example, if 5 low samples are tested, then where cβ =1.645/(1-1/(4f)) is derived from the 95th percentile of the standard Gaussian distribution (and the correction factor), which is applied because the SDS is a biased estimate for the population standard deviation σS LOD Determination Example sqrt(0.0119) cp = 1.645/(1-1/(4xf)) = 1.645/(1-1/4x60) = 1.6519 LODt = LOB + cβ x SDs 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 Expected MEAN STD %CV Bias STD^2 SDs cbeta LOB/LON LOD L1 L2 L3 L4 3.28 3.20 3.34 3.37 3.22 3.30 3.20 3.30 3.02 3.22 3.19 3.36 3.26 3.39 3.22 3.21 3.20 3.26 0.09 3 5.53% 0.0085 0.1092 1.6519 1.2 1.38 1.94 2.02 1.97 2.12 2.13 2.01 1.96 1.95 2.19 1.99 2.15 2.11 2.19 1.94 1.98 2.10 1.92 2.05 0.09 4 12.68% 0.0083 1.44 1.42 1.51 1.66 1.51 1.50 1.54 1.90 1.53 1.45 1.54 1.90 1.62 1.62 1.48 1.51 1.60 1.57 0.14 9 -2.90% 0.0209 1.40 1.43 1.49 1.46 1.41 1.40 1.36 1.14 1.31 1.27 1.36 1.25 1.20 1.25 1.41 1.28 1.37 1.34 0.10 7 -3.25% 0.0100 3.01% 0.0119 SD2S = (15*0.0085 + 15*0.0083 +15*0.0209 + 15*0.01) / 60 Considerations Using 4PL Conrad P. Quinn, Vera A. Semenova, Cheryl M. Elie et al (2002). ¾ When 4PL is used to model the characteristic curve for the standard data two estimates of LOD can be obtained: the MDC and the RDL. The position of the LOB with respect to the MDC and the RDL will determine the true LOD Definition of MDC and RDL ¾ The minimum detectable concentration (MDC) is the concentration of the analyte corresponding to the interpolated intersection of the lower asymptote of the upper 95% confidence interval (95% CI) with the 4-PL fit of the standards data ¾ The reliable detection limit (RDL) is the concentration of the analyte corresponding to the interpolated intersection of the upper 95% CI asymptote with the lower 95% CI of the standards data LOD Design and Analysis ¾ At a minimum, data from at least 30 reference curves shall be used to estimate the MDC and the RDL ¾ If the LOB is an OD value, it should be converted to the analyte concentration by using the standard curve calibration factor. If the LOB ≤ MDC then the LOD = MDC If the MDC ≤ LOB ≤ RDL then the LOD = RDL LOD Determination Example LoD Antigen Number of curves Number of Concentrations LoB (μg/mL) MDC RDL Tetanus 60 8 0.000 0.00031 0.00045 LoD (μg/mL) 0.0016 LOQ Experiment Design ¾The LOQ is the lowest amount of analyte in a sample that can be quantitatively determined with a stated acceptable precision and accuracy, under stated experimental conditions ¾The test results from the LOD study can be used to estimate the LOQ. At least 10 low levels samples with concentrations in the ranging from the LOD to approximately 4xLOD shall be tested at minimum of 5 to 10 times by at least two technicians in a minimum of two days or runs LOQ Estimation ¾ The procedure described for the estimation of the LOD will be used to determine the LOQ. The estimated LOQ is obtained as: LOQ = LOD + cβxSDS where SDS is the pooled estimate of the standard deviation of the low samples in the range of the LOD to 4xLOD after checking the statistical assumption of homogeneity of variance The LLOQ is estimated during Qualification/Prevalidation experiment!!! LLOQ Challenge During Validation WARNINGS!!! LLOQ cannot be estimated during validation experiments ¾A minimum of ten validation samples ranging from below the LOD to four times the LOD will be tested ¾Each sample will have a minimum of 10 independent determinations generated as described by the method ¾Verification of the LLOQ by inspection of precision profile (%CV) for point-at-which the assay deviates from acceptable performance (i.e. %CV ≤ 20%) LLOQ Challenge Example LLOQ – Precision Profile from <7 to 60 EU/ml SAMPLE # MEAN STANDARD DEVIATION CV (%) 4 13.41 2.7393 20.43 1 13.41 0.6820 5.08 5 14.07 3.7958 26.97 2 15.75 1.8806 11.94 3 15.83 1.7192 10.86 6 16.63 2.0629 12.40 Antigen Number of Reference Curves AAA 10 Test for Homogeneity P-value Conclusion LoD (EU/mL) 0.0011 Fail 5.77 LoQ SDs Cβ or DS LoQ (EU/mL) - 8.88 14.61 LLOQ – Precision Profile from <15 to 60 EU/ml SAMPLE # 9 5 4 8 3 7 10 2 6 1 N 8 13 15 15 15 14 15 15 14 15 MEAN 12.47 16.08 24.61 24.64 32.38 39.16 42.08 48.26 51.62 60.22 STANDARD DEVIATION 2.3343 2.7185 3.6272 3.1005 5.6747 3.8095 5.3051 7.5851 5.6713 5.9099 CV (%) 18.72 16.91 14.74 12.58 17.52 9.73 12.61 15.72 10.99 9.81 LLOQ Considerations for Functional Assays ¾ In functional assays LLOD and LLOQ are identical ¾ The LLOQ is the lowest level of antibody present in a sample that can be consistently detected with suitable precision and accuracy by applying the test procedure ¾ 5 to 10 low human sera (non-specific, e.g. sheep sera) can be tested a minimum of 11 times to confirm the LLOQ Statistical Methods for Functional Assays ¾Method 1: Wood and Durham (2000) – The probability that the maximum ratio of two distinct titers (obtained in the blind) on the same specimen will not exceed 2 ¾Method 2: Median Method – The percent of the results within one 2-fold dilution from the median titer ¾Method 3: Replicate within 4-fold range – The probability that the maximum ratio of two distinct titers on the same specimen will not exceed 4 Functional Assays Sample Precision ¾For the median method, a sample or a specimen with a given targeted titer is reproducible if “no more than 10% of the sample tested would have titers outside of a two-fold difference from their median titer upon repeat testing”. ¾For the probability method, a sample with probability greater or equal to 0.9 is deemed to be acceptably reproducible. Comparison of Precision Method Acceptance Criterion > 80% of the samples have no more than 10% of titers outside of a two-fold difference from their median titer upon repeat testing > 80% of the samples have precision probability > 0.9 Example LLOQ Estimation During Qualification Experiment 1:20 is the level of antibody present in a sample that can be consistently detected with suitable precision and accuracy by applying the test procedure The LLOQ of this assay is can be chosen to be 20 LLOQ Challenge for Functional Assays During Validation ¾A minimum of ten validation samples ranging from below the LOQ to four times the LOQ will be tested ¾Each sample will have a minimum of 11 independent determinations generated as described by the method ¾Verification of the LLOQ by inspection of precision profile for point-at-which the assay deviates from acceptable performance (i.e. (>=90% of results are within ± 2-fold of the Median) Example of LLOQ Challenge During Validation Titer Median Percent of results within ± 2-fold of the Median 1 40 40 40 80 40 40 40 20 80 40 40 40 100% 2 80 20 40 40 40 80 40 20 20 20 40 40 100% 3 40 40 40 40 20 40 40 20 20 40 40 40 100% 4 80 160 80 80 80 80 160 160 80 80 80 80 100% 5 20 40 40 20 20 20 20 40 20 20 40 20 100% 6 10 40 10 40 10 10 20 10 10 40 <10 10 73% Sample 1 2 3 4 5 6 7 8 9 10 11 Verification of the LLOQ (1:20) by inspection of precision profile for point-at-which the assay deviates from acceptable performance (i.e. >=90% of results are within ± 2-fold of the Median) Conclusion ¾The experiment design and statistical method used for the determination of LOD and LOQ is dependent on the assay type (e.g., ELISA, Functional or PCR) ¾Appropriate determination of LOD/LOD is increasingly becoming a regulatory concern for calculation of sero-conversion, sero-protection or geometric mean when licensing a vaccine or drug products Reference • • • • • • • • • • • Code of Federal Regulation - 21 CFR 211.165(e), 211.194(a)(2) Official Journal of the European Union - Commission Directive 2003/94/EC ICH Harmonized Tripartite Guideline – Validation of Analytical Procedures: Text and Methodology – Q2 (R1) – October 1994 – November 1996 European Pharmacopoeia – Technical Guide – December 1999 United States Pharmacopoeia - <1225> Validation of compendial procedures FDA Guidance for Industry – Analytical Procedures and Methods Validation – Draft Guidance, August 2000 FDA Guidance for Industry – Bioanalytical Method Validation – May 2001 NCCLS – Protocols for Determination of Limits of Detection and Limit of Quantitation; Approved Guideline – NCCLS document EP17-A – Clinical and Laboratory Standards Institute Volume 24, Number 34, October 2004 Conrad P. Quinn, Vera A. Semenova, Cheryl M. Elie et al (2002). “Specific, Sensitive, and Quantitative Enzyme-Linked Immunosorbent Assay for Human IgG Antibodies to Anthrax Toxin Protective Antigen” Emerging Infectious Disease Vol. 8 (10). Dominique Pifat, Assay Validation Presentation R.J. Wood & T. M. Durham, CDC, Atlanta, Georgia (2000). Reproducibility of Serological Titers, Journal of Clinical Microbiology, 1980 p. 541-545