06-10. Disc10a_Chem400_Spr13_skeletal
advertisement
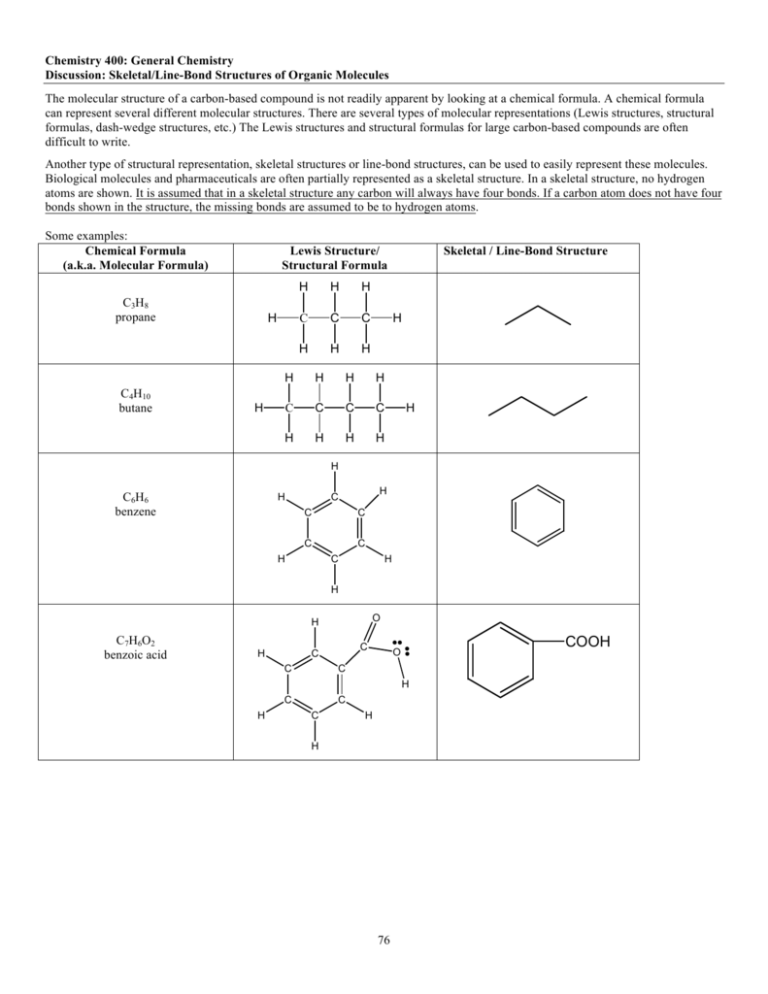
Chemistry 400: General Chemistry Discussion: Skeletal/Line-Bond Structures of Organic Molecules The molecular structure of a carbon-based compound is not readily apparent by looking at a chemical formula. A chemical formula can represent several different molecular structures. There are several types of molecular representations (Lewis structures, structural formulas, dash-wedge structures, etc.) The Lewis structures and structural formulas for large carbon-based compounds are often difficult to write. Another type of structural representation, skeletal structures or line-bond structures, can be used to easily represent these molecules. Biological molecules and pharmaceuticals are often partially represented as a skeletal structure. In a skeletal structure, no hydrogen atoms are shown. It is assumed that in a skeletal structure any carbon will always have four bonds. If a carbon atom does not have four bonds shown in the structure, the missing bonds are assumed to be to hydrogen atoms. Some examples: Chemical Formula (a.k.a. Molecular Formula) Lewis Structure/ Structural Formula C 3H 8 propane C4H10 butane H H H H C C C H H H H H H H H C C C C H H H H H Skeletal / Line-Bond Structure H H C 6H 6 benzene H H C C C C H C C H H O H C 7H 6O 2 benzoic acid H C C C C C C COOH O H H C H H 76 1. Draw a skeletal structure for the following compounds. Also, build each molecule with the molecular modeling kits. Pay particular attention to the angles and rigidity (or lack thereof) of a single and double bond bond. CH3CH2CH2CH2CH2CH3 CH2=CHCH2CH3 CH3CHCH2CH2CH2CH3 | CH3 CH3CHCH2CHCH3 | | OH CH3 2. Draw the structural formula/Lewis structure for the following substances. OH 3. One of the chemical components of DNA is cytosine (skeletal structure shown below). A. Add lone pairs to the nitrogen and oxygen atoms. B. What is the total number of lone pairs? C. What is the molecular formula for cytosine? NH2 N O N H 77 4. For the given line-bond structures, draw the corresponding structural formula/Lewis Structure, add lone pairs to the oxygen and nitrogen atoms, and then determine the molecular formula for the compound and list the number of lone pairs. Formula Skeletal Line-Bond Structure Structural Formula/Lewis Structure (# LP) 2-amino-3-methyl benzyl alcohol NH2 CH2OH Droxidopa (antiparkinsonian) OH COOH HO NH2 HO 5. For each of the following formulas, draw the skeletal structure. A. Fatty Acid: decanoic acid or capric acid O H H H H H H H H H H O C C C C C C C C C C H H H H H H H H H H B. Amino Acid: phenylalanine H H H H H N H C C C C O H C C H O C C H C H H C. Hexadecane CH3(CH2)14CH3 D. Heptadecane: CH3(CH2)15CH3 78 6. For each of the following molecules, draw the full Lewis structure structure. A. Triglyceride: glycerol reacted with 3 long-chain fatty acids O H2 C O O H2 C O O H2 C O B. Phospholipid: O O N + O P O O O O O 79 7. Structural isomers are molecules that have the same molecular formula but different bonding patterns of the atoms. Draw skeletal structures of: A. 3 structural isomers of C5H12 B. 5 structural isomers of C5H10 (different positions of the double bond are different molecules) 80