Geological Dating part 2
advertisement

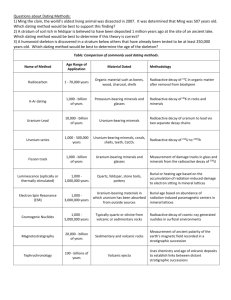
Geological Dating Clocks and Calendars Clocks in General • Clocks define time intervals. They tick. • We count ticks to measure elapsed time and to define our time scale • Ticks can be generated accurately using pendulums, oscillating balance wheels, oscillating crystals and oscillating electrons in an atom. • Longer ticks are derived from Earths rotation and orbit. Geological Clocks • An ideal geological clock: – Must run for many millions of years – Should not need calibration or re-setting – Must have a tick that is not affected by temperature, pressure, mechanical movement or chemical environment – Must count number of ticks over a long interval Radioactive decay, a signal from an atomic nucleus, meets these requirements. Measurement of the number of stable decay product atoms integrates the ticks. Geological Column • The study of fossil plants and animals and their associated strata, acceptance that the most recent sedimentary strata will be on top and appreciation of evolution allowed geological columns to be constructed. • The steps in the geological column represented ill-defined events, not time steps. • The origin was missing. No life, no fossils, no biological calibration ar bottom of column. Geologic Column Timescale The lowest segment was called pre-cambrian. There was no origin. The boundaries between periods are still argued over. A worldwide standard is probably impossible since temperature and climate histories vary. Old igneous rocks could not be dated and the mechanics of their formation can invalidate arguments based on stratification. Radioactive Decay 1 • For a given atom, the probability of decay is constant. • A large assembly of atoms exhibits a constant decay rate λNt where – λ is a constant for an atom, Nt is the number of atoms of that atom present at time t • Radioactive decay is therefore exponential Nt = N0e – and the time required for half to decay is 0.693/λ. Half life is more easily visualised than a reciprocal unit. Radioactive Dating Typically one measures the number of active parent and stable daughter atoms present in a mineral at a time t, the present. Since the number of stable daughter atoms, D*, is always the same as the number of parent decays, one can write an expression for the number of parent atoms when t = 0, the date we seek. This leads us to: D* = Nt(eλt – 1) where D* and Nt are measurable. This is the geochronometry equation. Measured Quantities • Mass spectrometry is favoured analytical method as sensitivity improves. • Mass spectrometers measure ratios of similar masse most accurately. • So geochronometry equation looks like this in practice. 86Sr is stable, natural reference isotope. 87 𝑆𝑟 86 𝑆𝑟 = 87 𝑆𝑟0 86 𝑆𝑟 + 87 𝑅𝑏 86 𝑆𝑟 (𝑒 𝜆𝑡 - 1) Radiometric dating: • Radiometric dating is most successful when the number of parent and daughter isotopes are changing rapidly with time. The graph shows that after 4 half lives the method becomes very much less sensitive as growth and decay curves flatten. Hence need for a toolbox of isotope pairs. Sources of Natural Radioactivity • The creation of heavy atoms during stellar explosions that release many neutrons. • The heaviest atoms, and a few lighter isotopes, are unstable, so radioactive. Some survive in the universe. • Secondary cosmic ray packets contain neutrons that can react with earthbound nuclei to produce unstable atoms e.g. 14C. 14 7𝑁 + 10𝑛 14 6𝐶 + 11𝐻 nuclear mass nuclear charge (protons) Atom Long lived naturally occurring Isotopes used in Dating (a = years) 238 Uranium Decay Chain Cosmogenic Radionuclides When can one use Radiogenic Dating • To date compounds of radioactive elements eg: Uranium minerals, thorium deposits etc. • To date any undisturbed mineral that contains a radioactive atom as an embedded impurity. • Uranium and thorium are widely distributed. • Zircon is a widely distributed, but insoluble, impurity present in many igneous rocks able to sequester uranium and thorium. It’s a tight, easily recognised and removed box. A good marker, but it can leak lead. Dating with Long lived isotopes What is T0 ? • Date of supernova explosion that created the heavy atoms used in dating? • Date the explosion debris solidified? • Date Earth formed, whatever that means? • Date this particular crystal formed from a solution or a melt and excluded daughters? Caveats and Assumptions • Number of parent and daughter atoms in specimen changed only by decay of parent to daughter. No loss by ejection, evaporation or solution. No gain by contamination from environment. • Isotope ratios in parent were not affected by fractionation at the time of mineral formation. • No metamorphosis that affects composition of rock specimens. Problems with Reality • D0 not zero at time of specimen formation • Loss of Lead from chemical reaction and solution • Zeolite lattice does not like lead, so loses some. • Specimen histories easily vary over a few million years • Metamorphism causes re-distribution of materials in rocks. Restarts clock at best. • You do not choose available markers D0 not Zero • Non-zero D0 can be dealt with if several samples believed to be of same age are analysed for remaining parent and daughter. • A plot of Daughter atoms (y) versus Parent atoms (x) has a slope of (𝑒 𝜆𝑡 - 1) and intercept D0. • Variation of D0 yields information about history. • Scope for argument Cosmogenic Dating The measured quantity is the radioactivity (λNt) of an isotope whose accumulation in a mineral or organic material has ceased due to chemical isolation or death of the organism. For dating to work: The rate of production of the isotope must have been constant for a long time for radioactive equilibrium to be established. The composition and intensity of cosmic ray incidence must have been constant . No interfering sources or fractionation. Carbon Dating • Sources of interference: – Solar flares – Neutrons from nuclear bomb testing that react with nitrogen in air to enhance 14C – Combustion of carbon of various ages • Sources of fractionation: – Mass difference 12C and 14C so – All chemical reactions from photosynthesis to cellulose and tissue production. – Dissolution of CO2 and CH4 in water Radio-carbon dating: Validation of Carbon Dating • Many sources of error make calibration and validation necessary. • One method is tree or coral ring analysis, since age of ring is known from the ring count. • Another is use of other short lived cosmogenic nuclide pairs whose environment is different or parts of a longer sequence 234U/230Th/226Ra • Counting 16O/18O switches Dendro-chronology: • Tree rings are normally formed annually and indicate growth of trees over many years sometimes, hundreds or even thousands – up to 9000 years especially using Britstlecone Pines. Radiocarbon Dating Calibration Curve Radiometric dating - Summary