electrical potential energy
advertisement
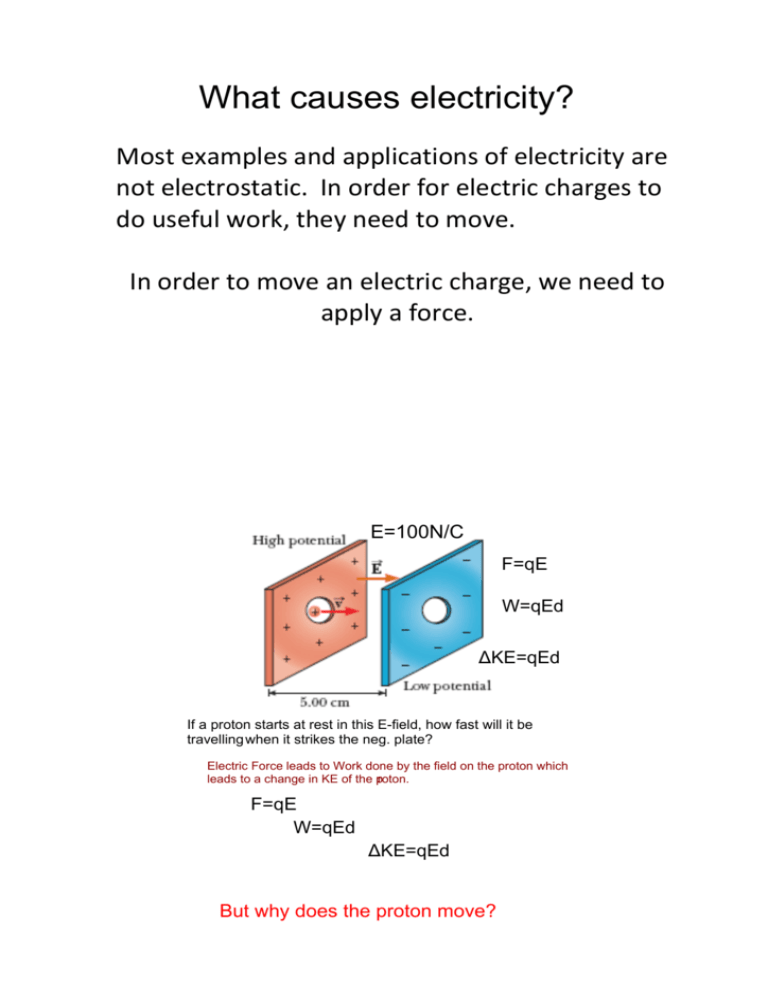
What causes electricity? Most examples and applications of electricity are not electrostatic. In order for electric charges to do useful work, they need to move. In order to move an electric charge, we need to apply a force. E=100N/C F=qE W=qEd ΔKE=qEd If a proton starts at rest in this E­field, how fast will it be travelling when it strikes the neg. plate? Electric Force leads to Work done by the field on the proton which leads to a change in KE of the proton. F=qE W=qEd ΔKE=qEd But why does the proton move? Electrical Potential Energy • What is needed in order to move a charge against the influence of an electric field? An external force • If work is done on the charged particle by this force to change the position, what happens to its energy? It increases!!! (Law of Conservation of Energy) • What will the external Electric field do to the charged particle when released? • What will the work done on the charged particle by the electric field do to the energy? Decrease it. Electrical Potential Energy Since this work changes a charge’s position in the electric field, the type of energy it gains is potential, specifically electrical potential energy (PEE) Work done against the field always increases PEE Work done by the field always decreases PEE Electrical Potential Energy • Draw field diagram for an electric field surrounding a positive charge (proton) • Draw field diagram for an electric field surrounding a negative charge (electron) • For a small, positive test charge… where would it have a large PE? A small PE? • Would the PE be numerically the same for a slightly larger (or smaller) test charge? Cells and batteries do work on charged particles by exerting a force on the charges to separate them. Large amounts of charge are separated, resulting in large amounts of Potential NRG. How do we now deal with calculating the NRG due to not just two charges but multiple opposite and like charges being placed in positions of increased NRG?........ Electrical Potential • Electrical Potential Energy depends on the size of the test charge. • If we want to get a picture for the energy independent of the test charge, we need to look at electrical potential Electrical Potential (V): potential energy per unit charge PEE V = q 0 Units: joules per coulomb (J/C) or volts (V) Electric Potential is commonly known as Voltage Potential Difference Electric potential itself is not useful, only the change in electric potential provides us with useful information. • Potential Difference (ΔV): change in electrical potential energy per unit charge ΔV = Vf – Vi • The potential difference between any two points A & B equals the work done against the field in moving a positive test charge from A to B with no acceleration: ΔV = WAB / q0 and ΔV = ΔPEE / q0 The work done is independent of the path taken in a uniform E­field: ΔV=qEd=Ed q Potential Difference • For a proton or an electron (qe) a change in potential of 1 V, produces a change in PEE of 1.6 x 10‐19 J While very small in size, many atomic phenomena involve energies of this order of magnitude. A reasonable unit is needed in order to report this energy. ... e rop exp d l i o e Th riment • Electron Volt (eV): amount of energy corresponding to an electron falling through a potential difference of one volt 1 eV = 1.6 x 10‐19 J











