Chapter 8
advertisement

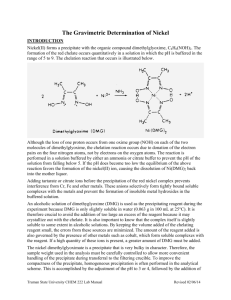
Intro Lab Methods Chapter 8 Gravimetric Analysis Gravimetric analysis is the use of weighing to determine the amount of a component in your sample. Gravimetric analysis, or gravimetry is normally performed either as a : • loss of volatiles procedure where the sample is heated to release volatiles such as moisture or organic vapours and the change in mass is used to calculate the volatile content, or • precipitation or separation procedure where a component in the sample is isolated or recovered in some form and this is weighed to complete the analysis. Loss of volatiles gravimetric analysis All loss of volatiles analysis is performed by a similar set of simple steps: 1. weigh your fresh sample 2. heat to remove volatiles 3. reweigh to measure mass loss 4. calculate percentage loss of mass or percentage residual mass In practice, many problems can arise if you consider what might go wrong in each of the steps. You need to: (i) weigh enough sample to keep accuracy high but not too much so as to hinder vapour loss (ii) heat but not for too long or too hot or too cold (iii) cool without additional losses or pick-up of volatiles (iv) reweigh without losses or gains (v) be sure the container does not change weight during the procedure. Heating to constant weight is the chief method used to ensure the container is not altered during treatment. Empty containers are subjected to the same treatment as the sample before any analysis and if their masses remain constant, then you can be sure they have been heated to constant weight and their empty mass is correct. Precipitation or separation gravimetric analysis Precipitation or separation gravimetric analysis follows these simplified steps: 1. weigh your fresh sample 2. treat to isolate component of interest 3. measure mass of pure, recovered material 4. calculate percentage of component The treatment required to isolate the component can cover a wide range of techniques and procedures including: (i) digestion with powerful reagents such as enzymes, acids or oxidants (ii) buffering to ensure conditions are suitable for recovery of the analyte (iii) addition of chemicals designed to selectively and quantitatively capture the component of interest. (iv) use of prolonged heating or contact with solvents such as occurs with Soxhlet extraction or Dean and Stark moisture determination. 65 | P a g e Intro Lab Methods Validation of Gravimetric Analysis A requirement with all laboratory testing is some evidence or assurance of the reliability of your answer. This evidence comes in four major ways: 1. replicate analysis 2. control or standard analysis 3. literature values 4. alternative testing procedure This set of practicals is very time consuming and it will often be the case that a number of the practicals will be scheduled for the one session. It is up to the student to read the methods and practice good time management skills in order to complete all the tasks by the scheduled date. The full methods for each of the required tasks are given on the worksheets. Practical work 8.1 Moisture content by an oven-drying technique Caution: This practical involves the use of drying ovens and high temperatures. It is important that you take care when handling all equipment. The treatment for a heat burn is to run cold water over the affected area and inform the teacher that you have been burnt. Practical work 8.3 Moisture content of difficult samples Practical work 8.4 Ash determination Caution: This practical involves the use of the bunsen burner and the muffle furnace. You must be extremely careful when working and be observant of other workers in the laboratory. Practical work 8.5 Total dissolved solids Caution: This practical involves the use of steam baths. Steam burns are very painful. Be extremely careful when working with or near the hot steam baths. Practical work 8.6 Gravimetric determination of sulfate in bore water This practical will develop your skills with samples, methods and equipment used to selectively precipitate an analyte, then purify and isolate it for weighing. Caution must again be exercised with steam, drying ovens etc. 66 | P a g e Intro Lab Methods Student Name: Practical: Moisture content by oven-drying Practical Number: 8.1 Date Performed: Date Submitted: Text book References Procedure: 1. Accurately weigh two clean labelled weighing dishes complete with lids. (The weighing dishes have been numbered and heated to constant mass). 2. Accurately weigh about 2 g of the allocated food product into each dish. 3. Place the weighing dishes in the drying oven with the lids off. Leave the samples here for 30 mins. 4. Remove the dishes and the lids, carefully, and place in a desiccator. 5. When cool accurately reweigh the dishes plus lids. 6. Repeat steps 3 to 5 until constant mass is obtained (if necessary). 7. Clean the dishes thoroughly. Results: Sample Sample 1 Sample 2 Name of food item analysed Constant mass of empty container Mass container + food Mass food Mass of container + food after heating Mass of moisture lost % moisture = mass moisture lost x 100 sample mass Questions: 1. Why shouldn’t containers be handled with the fingers during the analysis? 2. Why are the samples placed in a desiccator when removed from the drying oven? 67 | P a g e Intro Lab Methods Student Name: Practical: Ash determination Practical Number: 8.4 Date Performed: Text book References Date Submitted: Procedure: This practical is designed to determine the ash or mineral content of a food product after heating in a muffle furnace. This heating removes all organic matter. 1. Accurately weigh two prepared crucibles and lids. (The crucibles and lids have achieved constant mass). 2. Weigh into each crucible accurately 1g of the allocated food product. 3. Place the crucibles with samples on a pipe-clay triangle with the lid slightly ajar, gently heat the sample with a bunsen burner (as shown by your teacher). 4. When the sample has stopped smoking, place the crucible and lid in a muffle furnace at 550oC for at least two hours (the lab staff will remove your sample and place it in a desiccator). 5. Collect your sample from the desiccator and reweigh. Results for food item analysed (name your food item in this box): Sample I.D. code 1 2 ID of crucible and lid Mass of empty crucible + lid Mass crucible + lid + food Mass crucible + lid + food after ashing in muffle Mass of original food Mass of ash residue % Ash = mass of residue x 100 mass of food Questions: 1. Why must containers removed from a muffle furnace be air-cooled before placing in a desiccator? 2. What is the purpose of the pre-charring step in the ash determination? 68 | P a g e Intro Lab Methods Student Name: Practical: Total Dissolved solids (TDS) Practical Number: 8.5 Date Performed: Date Submitted: Text book References Procedure: 1. Label 2 clean and dry evaporating basins and weigh them on a top-pan balance. 2. Pipette a 50 mL aliquot of the allocated water sample into each basin. 3. Place the basins on a water bath and allow to evaporate to dryness. 4. Remove the basins from the water bath and dry them on the outside with a paper towel. 5. Place basins in drying oven for one hour minimum. 6. Wafter cooling, weigh the basins plus residue. Results: Sample 1 Sample 2 Weight of basin Volume of sample added Weight of basin + residue at constant weight Mass of residue TDS % (w/v) = mass of residue x 100 volume of sample Questions: Why is this determination carried out in evaporating basins rather than in beakers? 69 | P a g e Intro Lab Methods Student Name: Practical: Gravimetric determination of sulfate in bore water Practical Number: 8.6 Date Performed: Date Submitted: Text book References Procedure: 1. Pipette 25mL of bore water, in duplicate, into 400mL beakers and dilute each to about 200 mL with distilled water. Prepare a blank sample by pipetting 25 mL of distilled water into another 400 mL beaker and treating it identically to the bore water samples 2. Add about 5 mL of 2 M HCl to each beaker 3. Place a stirring rod into each beaker and heat to boiling on a hotplate 4. Remove from the heat and immediately after boiling stops, slowly add about 12 mL of 5% BaCl2 with constant stirring to form the BaSO4 precipitate. 5. Allow the precipitate to settle for a few minutes and check for completeness of precipitation by adding a further 1 mL of BaCl2 solution 6. Cover the beakers with watch-glasses and digest them for one hour, at a temperature just lower than the boiling-point 7. Dry at least three cleaned and labelled sintered glass crucibles in an oven at 110oC for 30 minutes. Cool them in a desiccator and weigh them on an analytical balance. Record their mass on the worksheet. 8. Filter each digested solution through its own oven-dried sintered glass crucible as demonstrated by the teacher. 9. Wash the precipitate 10 times with 5 mL of water and drain well between washes. 10. Place the crucibles into an oven at 110oC until they achieve constant mass. Results Sample 1 Sample 2 Blank mL of bore water sample Crucible ID Mass of empty crucible (g) after heating to constant weight Mass of crucible + residue (g) at constant weight Mass of residue (g) as BaSO4 **Mass of SO4 (g) SO4 concentration in g/L Calculate your results for “g of sulfate” per litre of bore water. **You will need to correct for the Ba which was not present in the bore water but it has contributed to your mass of isolated residue (see text book for a hint). 70 | P a g e Intro Lab Methods GRAVIMETRIC DETERMINATION OF NICKEL This method involves converting nickel ions which are soluble in water into a complex which has reduced solubility at an increased pH. The procedure involves the use of a complexing agent dimethylglyoxime and strict pH monitoring to form an insoluble solid. The complex formed is Nickel dimethylglyoxime. The analysis is gravimetric as the precipitate formed is collected and weighed to determine the mass of nickel. SAFETY 1.The techniques involves digesting the solution over a steam bath. Remember steam and skin do not go well together. 1. Ammonia has a strong odour and work with ammonia should be carried out in the fume hood. When smelling do not inhale deeply (Use the wafting technique as demonstrated by your teacher.) 2. Concentrated HCl and skin to not go well together. 3. The sintered glass crucibles will be hot when removed from the drying oven PROCEDURE Clean two sintered glass filter crucibles with dilute HCl and dry in a 110oC oven. Pipette out two 20 mL samples of the supplied unknown into two 600 mL beakers Dilute to about 150 mL with purified water Add approximately 3g of citric acid to each solution and stir to dissolve. Leave the glass stirring rod in the beaker 5. Slide a piece of red litmus paper down the side of the beaker until it is mostly immersed in the solution, but sticks to the side. 6. Add 5 M ammonia slowly until the litmus turns blue, and then add concentrated HCl slowly until the paper returns to red. Add 2 mL more of concentrated HCl. Remove the litmus paper. 7. Heat the beakers on a hotplate to about 80oC but do not allow to boil. 8. Remove the beakers from the hot plate and slowly add 50 mL 1% dimethylglyoxime (DMG) solution with stirring. If a red precipitate begins to form, add concentrated HCl dropwise until it redissolves, and then continue to add the DMG solution. 9. Add 5M ammonia slowly with stirring until a red precipitate forms and the solution smells strongly of ammonia. 10. Stir well and stand on a steam bath for 30 minutes. 11. Check that the solution still smells of ammonia, (add extra if necessary) 12. Add 2 mL extra DMG using a plastic pipette, and stir well. 13. Cool the beakers to room temperature and filter the solutions through the previously dried and tared sintered glass filter funnels. 14. Wash the precipitate in the crucible 3 to times with water 15. Finally wash the precipitate once with 20-30 mL of 30% aqueous ethanol, which dissolves any remaining DMG from the precipitate. 16. Place the crucibles in an oven at 110oC for at least 2 hours. 17. The crucibles should be cooled in a desiccator and then reweighed. 1. 2. 3. 4. 71 | P a g e Intro Lab Methods RESULTS Sample 1 Sample 2 Volume solution taken Mass of sintered glass crucible Mass crucible + Ni(DMG)2 CALCULATIONS 1. Ni2+(aq) + 2DMG Ni(DMG)2(s) 2. Moles Ni2+ in original solution = moles of Ni(DMG)2 formed = mass Ni(DMG)2 . 288.88 3. Mass nickel in original solution = moles of nickel x Formula mass nickel Sample 1 = Sample 2 = Ave = 4. %w/v of nickel in the sample = mass of nickel in sample x 100 vol of sample QUESTIONS 1. Why was it important to limit the amount of nickel in the sample by only taking 20 mL? 2. Why was the precipitate washed in aqueous ethanol? 3. Why was a sintered glass crucible used rather than filtration through paper? 72 | P a g e