Nomenclature of pyroxenes - Mineralogical Society of America
advertisement

American Mineralogist, Volume 73, pages I I 23- I I j 3' I 988
Nomenclatureof pyroxenes
Subcommittee on Pyroxenes
Commission on New Minerals and Mineral Names
International Mineralogical Association
N. Momvroroo
Chairman
Department of Geology and Mineralogy, Kyoto University, Kyoto 606' Japan
Members
Subcommittee
J. F.LsRrns(France),A. K. FnncusoN (Australia)I. V. GrNzruRG(USSR),M. Ross (U.S.A.),
F. A. Srmnnr (Germany),J. ZussMAN (U.K.)
NonvotingMembers
K. Aoxr (Japan),G. Gorr.l'nor (Italy)
Ansrucr
This is the final report on the nomenclature of pyroxenes by the Subcommittee on
Pyroxenesestablishedby the Commission on New Minerals and Mineral Names of the
International Mineralogical Association. The recommendations of the Subcommittee as
put forward in this report have been formally acceptedby the Commission. Accepted and
widely used names have been chemically defined, by combining new and conventional
methods, to agreeas far as possiblewith the consensusof presentuse. Twenty names are
formally accepted,among which thirteen are usedto representthe end membersof definite
chemical compositions. In common binary solid-solution series,speciesnames are given
to the two end members by the "500/oruIe." Adjectival modifiers for pyroxene mineral
names are defined to indicate unusual amounts of chemical constituents.This report includesa list of 105 previously used pyroxene names that have been formally discardedby
the Commission.
present use. Two kinds of adjectival modifiers are used:
one to specify a part of the compositional range shown
The Subcommittee on Pyroxeneshas, after a thorough
by a mineral that forms a wide solid solution [e.g.,magevaluation ofthe group ofpyroxene minerals, presented
nesium-rich (or Mg-rich) augiteand iron-rich (or Fe-rich)
its recommendationsfor a new classificationand nomenaugitel; the other to specifo elemental substitutions that
clature to the Comrnission on New Minerals and Mineral
are not essentialconstituents (e.g., titanian augite). The
Names (hereafter abbreviated as CNMMN). These recCNMMN has formally discredited 105 previously used
ommendations have been approved by the Commission
pyroxene names-mostly synonyms, obsolete or almost
by a formal vote (May 20,1987).
unused, or recommendedfor rejection.
The classification and nomenclature of the pyroxenes
General publications dealing with the pyroxene group
have been largely based on their crystal chemistry. In
Rock-Forming Minerals (Deer et al., 1978),Mininclude
practice the chemical content of the pyroxene formula
Society of America Special Paper 2 (Papike,
eralogical
unit calculated to six oxygens,or to four cations (Vieten
MSA Revkws in Mineralogy, volurne 7 (Prew1969)
and
and Hamm, 1978),is essentialfor the classification.This
which provide referencesto the voluminous
1980),
itt,
formula unit correspondsto one-quarter of the unit cell
literature.
for the monoclinic pyroxenes and to one-eighth of the
unit cell for the orthorhombic pyroxenes.The basic prinCnvsr.lr, CHEMISTRYoF THE PYRoxENES
ciple adopted for amphibole nomenclature (kake and
are silicates that, in their simplest form,
Pyroxenes
Winchell, 1978) is to denote principal stoichiometriesby
singleSiO, chains oflinked SiOotetrahedra.Gencontain
generally well-establishednames, with adjectival modierally, small amounts of Si are replacedby Al and other
fiers to indicate the presenceof substantial substitutions
small cations. The repeat along the chain (c axis) comthat are not essentialconstituents of the end members;
prises two tetrahedra and is approximately 0.52 nm in
this principle has been followed as far as possible in the
length. The general chemical formula (formula unit) for
pyroxene nomenclature.
all pyroxenes'is M2MlTrOu, where M2 refers to cations
No new names have been introduced in the proposed
nomenclature. Accepted and widely used nameshave been
I In omphacite-P2/n,the Ml and M2 sitesare further divided
chemically defined by combining new and conventional
methods to agreeas far as possiblewith the consensusof into Mla and Mlb (for Ml) and M2a and M2b (for M2).
INrnooucrroN
0003{04v88/09 I 0-l I 23$02.00
tt23
n24
MORIMOTO:NOMENCLATUREOF PYROXENES
-3+
!e
Ti4+
1+
Cr..3+
?+
Ti-'
zt
A+
zn2+
tg2* -
tf2*
Fe2* -
2+
'
Fe-
l,t 12* -
Itr2+
I,i-
+
Na'
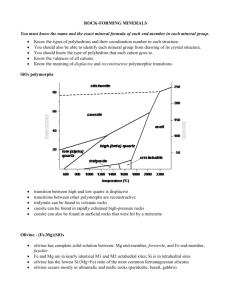
Fig. l. Flowchartfor idealsiteoccupancy
of cationsbetween
the T, Ml, and M2 sitesof pyroxenes.
Only representative
cations areincluded.Arrowsindicateorderoffilling ofsites.Real
site occupancyis usually slightly different from the ideal site
occupancy.
in a generally distorted octahedral coordination. Ml to
cations in a regular octahedral coordination, and T to
tetrahedrally coordinated cations.
Any pyroxene belongs to either the orthorhombic or
the monoclinic crystal system.There are two orthorhombic pyroxene types: orthopyroxene (Pbca) and orthopyroxene (Pbcn).,Only the former has been found in nature.
Monoclinic pyroxenes are called clinopyroxenes. Their
space groups are C2/c, P2,/c, and P2/n, depending on
their chemical composition and petrogenetichistory.
Throughout this report, the standardpyroxeneformula
is usedwith superscriptedarabic numerals (e.g.,Fe2*)referring to chargesand subscripted numerals (e.g., MgJ
referring to numbers of atoms.
In order to derive a pyroxene formula from a chemical
analysis, the calculation should be based on six oxygen
atoms when Fe2* and Fe3* are both determined. In microprobe analyses,only total Fe is determined, and the
option of calculating to four cations should at least be
permitted if not actually preferred. Vieten and Hamm
(1978) have shown that calculation to four cations will
be more reliable for microprobe analysesof the majority
of pyroxenes. Therefore, for microprobe analyses,it is
recommendedthat the componentsbe totaled to six oxy-
gens and four cations by adjusting the ratios Fe2*/Fe3*,
Ti4+/Ti3+,etc.
The standard pyroxene formula M2MlTrO6 contains
two tetrahedral sites. In the allocation of the cations to
obtain a pyroxene formula, the following procedure is
recommended:
1. Sum T to 2.000 using Sia*,then Al3*, and then Fe3t.
2. Sum Ml to 1.000 using all Al3* and Fe3*in excess
of that used to fill the T sites. If there is insufficient Al3*
and Fe3*to sum to 1.000,then add Tia*, Cr3*,V3+,Ti3+,
Zra*, Sc3', Zn2*, Mg2', Fe2*, and finally Mn,* until the
sum is 1.000.
3. Sum M2 using all Mg2*, Fe2*,and Mn2t in excessof
that used to fill the Ml sites. Then add Li*. Ca2*. and
Na* so that the sum becomes 1.000 or close to it. If the
sum is far from 1.000, one must be suspiciousabout the
results of the analysis.
A flow chart (Fig. l) gives a diagrammatic representation ofthe site allocation ofthe principal cations in pyroxenes. However, because the distribution of cations
among the Ml, M2, and T sites in a given pyroxene is
partly a function of temperature, the accurate site occupancy must be determined by structure determination.
The site occupancygiven in Figure I is called ideal site
occupancyto distinguish it from real occupancy.A method for classifuingpyroxenesby their ideal site occupancies has been proposedby Bokij and Ginzburg (1985). In
the present classifrcationof pyroxenes, the Ml and M2
sites are considered together as a single M site in order
to avoid the difference between the real and ideal site
occupancles.
Starting from the most common pyroxene formula,
M2(R'z-)MI (R2*)Tr(2R4*)O6,four coupled substitutions
are possible if one assumesmore than one Ra* in the T
site. They are listed in Table l, where the elements in
parenthesesare coupled substitutions.
Substitution I encompassesthe end members jadeite
(NaAlSirOu), aegirines (NaFe3*SirOu),kosmochlora
(NaCr3*SirO.,Ko), and jervisite (NaScSirOu,Je). Substitution 2 results in components such as NaFefrjTiftsi2o6,
but is less important than the other substitutions.
In substitution 3, the Al-Al couple is often referred to
as "Tschermak's component"; CaAlAlSiOu,in particular,
is called "calcium Tschermak's component." Substitution in esseneite,s
CaFe3*AlSiO.,is obtained by this type
of substitution. This substitution is also important in
3"Aegirine"is usedin preference
to "acmite" in this report.
"Aegirine"is in commonusagein theliteratureandis consistent
with the almostuniversaluseof "aegirine-augite"
for minerals
of intermediate
compositions,
though"acmite" haspriority by
14 years(Dana,1892).Commonpracticein experimentalpetrologyhasbeento usethe abbreviationAc for NaFe3*SirO6;
Ae
shouldnow be usedinstead.
4TheCNMMN, IMA, hasrecentlyvotedin favorof thename
"kosmochlor"insteadof "ureyite" for the pyroxeneofgeneralizedcompositionNaCrSirO..
2 Orthopyroxene(Pbcn)is stahleonly at elevatedtemperatures
5Esseneiteis a new pyroxenewith the compositionCafor a limited composition near MgSiOr.
Fe3*AlSiOu
Gable 2, no. l3).
tI25
MORIMOTO:NOMENCLATUREOF PYROXENES
TeeLe1. Fourcoupledsubstitutions.
of pyroxenesin the standardchemical
formulaR2+R2+R4+06
namesare given for the end-member componentsof substitutions 2 and 4.
Substitution
site
Examples
M1
Standardoccupancy
1
Substitution
R2*
R2*
2R4*
(R)
(R*)
2R4*
NAMES oF THE PYRoXENES
MrNBul
Twenty (20) mineral namesand their grouping
Na-Al
Na-Fe3+
Na-C13*
Na-Sc3*
R63(R33) 2R4*
Na-Oi4*/2)
(R"t,
(Rr+)Re
AI-AI
Fe+-Al
Cr3+-Al
R6i(R6i) (Rs+)R4+ (Ti4V2)-Al
The pyroxenesform extensive solid solutions by various types of ionic substitutions, some of which are described above. To cope with the problem of pyroxene
(R.)
Substitution
2
nomenclature, it is necessaryto subdivide the solid-soSubstitution
R2*
3
lution seriesinto rangeswith specifiedcompositions and
names. Whenever there is a complete solid-solution seSubstitution
4
R2*
ries betweentwo end members,it is customaryin mineral
'Shownby parentheses.
nomenclature to use only two names, and the division
betweenthem should be at AroBro(the "500/0rule"). However, this "500/orule" cannot be applied rigorously to the
"fassaite."6 Substitution resulting in CaTi3*AlSiOu was large groups ofpyroxenes that show wide rangesofcoureported by Dowty and Clark (1973) and Mason (1974) pled substitutions. This is particularly so when the minin pyroxenesfrom the Allende meteorite (Table 3, no. 4). erals concerned are abundant and widespread and have
In substitution 4, the component CaMgorTiS.SAlSiOu
is a historically establishednomenclature in mineralogical
found in some pyroxenes. There are a few instances of and petrologicalcircles.Taking this situation into considthe component of substitution 2 or 4 amounting to nearly eration, 20 acceptedand widely used names have been
500/0,as describedlater (Table 3). However, no particular adoptedas mineral speciesnamesof the pyroxenes(Table
2).
6"Fassaite"hasthegeneralformulaCa(Mg,Fe3*,Al)(Si,Al)rOu. The definition ofthe pyroxene specieshas been based
This namehasbeenrejectedasa formalnamein this report.
on 13 end members, or chemical components (given in
TnaLe2. Acceptedpyroxenemineralnamesandtheirchemicalsubdivisons
Mineral names
A. Mg-Fepyroxenes
1. enstatite (En)
2. ferlosilite (Fs)
3. clinoenstatite
4. clinoferrosilite
5. pigeonite
B. Mn-Mg pyroxenes
6. donpeacorite
7. kanoite (Ka)
C. Ca pyroxenes
8. diopside (Di)
9. hedenbergite(Hd)
10. augite
11. iohannsenite(Jo)
12. petedunnite(Pe)'
13. esseneite (Es)".
Composition as end member
MgrSirO6
Fee+Sir06
MnMgSi,O6
GaMgSi,O.
CaFe'*Sir06
NaAlSiro6
NaFe3+Si2O6
NaCre*Si2O6
NaScP*Siro6
Spacegroup
(Mg,Fe),Si,O6
Pbca
(Mg,Fe),StO€
nllc
(Mg,Fe,CaLSLO6
PJc
(Mn,Mg)MgSi,Os
(Mn,Mg)MgStO6
Pbca
PJc
Ca(Mg,Fe)St06
C2lc
(Ca,Mg,Fe),SirO6
C2lc
C2lc
C2lc
C2lc
(Ca,NaXR'z*,Al)StOo
(Ca,NaXR,*,Fes*)Si,O6
C2lc, Pln
C2lc
Na(Al,Fe3+)SirOs
C2lc
CaMnSiro6
CaZnSi,O6
CaFe#AlSi06
D. Ca-Na pyroxenes
14. omohacite
15. aegirine-augite
E. Na pyroxenes
16. iadeite (Jd)
17. aegirine (Ae)
18. kosmochlor(Ko)
19. iervisite (Je)t
F. Li pyroxene
20. spodumene (Sp)
Main composition as solid solution
C2lc
C2lc
LiAtsi,o6
A/ote:Name,abbreviation,and compositionare given for any pyroxenethat is used as an end memberot a pyroxenesolid solution; such end members
are printed in boldfacetype. Main compositionsare given for solid solutions. Space groups are also given.
- Petedunnitehas been determinedby Esseneand Peacor(1987)
to have the composition(CaopNao6Mno@Xzno3zMno
ieFq:frF4lrMgo,.XSi,sAlo6)00
by means of an electron microprobe.This mineral was approved as a valid species by the CNMMN, lMA, in 1983.
'-Esseneite has been determinedby
by
Cosca and Peacor(1987)to have the composition(Ca,oNaoorxF6tMgo,6AloqTio6F4.[r)(Si,,rAlor,)O.,
means of an electron microprobe.This mineralwas approved as a valid species by the CNMMN, lMA, in 1985.
by means of an
f Jervisite has been determined by M. Mellini et al. (1982) to have the composition (Nao€Caoo1F414troit(Sco6FqlsMgo,e)Siroo
electron microprobe.This mineralwas approved as a valid species by the CNMMN, lMA, in 1982.
tt26
MORIMOTO:NOMENCLATUREOF PYROXENES
bold face in Table 2) and the component CarSirOu(Wo).'
Theseend members are given the names of the minerals
whose compositions they most closely approximate. The
20 pyroxene speciesare grouped into six chemical subdivisions on the basis of the cation occupancyof the M2
sitesand crystal-chemicalsimilarity. This classificationis
a slight modification of the widely used schemeproposed
by Deer er al. (1978).
For the precise classificationof the pyroxenesinto 20
mineral species,however, the following characteristicsof
the pyroxenes must be considered. First of all, the MgFe pyroxenesand some of the Ca pyroxenesare the most
common rock-forming pyroxenes and form wide solid
solutions that cover the pyroxenequadrilateral ofthe ternary Ca,SirOu(WoFMgrSirOu (EnlFerSi.Ou (Fs) system.
Therefore, these pyroxenesare better treated together as
the Ca-Mg-Fe or "quadrilateral" pyroxenes.Second,Na
pyroxenesform continuous solid-solution serieswith the
Ca-Mg-Fe pyroxenes, forming the Na-Ca pyroxenes.
Third, donpeacorite and kanoite in the Mn-Mg pyroxenes,johannsenite, petedunnite, and esseneitein the Ca
pyroxenes, and spodumene are rare in occurrence and
unique in chemistry. For simplicity they are treated together as "other" pyroxenes.E
All the pyroxenesare thus divided into four chemical
groups for the purpose of broad classification:Ca-Mg-Fe
pyroxenes (Quad, 8), Ca-Na pyroxenes (Ca-Na, 2), Na
pyroxenes(Na, a) and other pyroxenes(Others, 6). The
abbreviations of the groups and the numbers of the accepted speciesare given in parentheses.Quad represents
"quadrilateral" for the Ca-Mg-Fe pyroxenes. The four
chemical groups are further divided into 20 mineral
speciesby using 12 components (the Wo component is
used for the Di and Hd components). The composition
rangesfor the acceptednames will be given later in this
report.
The pyroxene names may be qualified by one or more
adjectival modifiers according to defrnite rules described
later in this report to specifuirnportant (though relatively
minor) departures from the composition ranges. When
the composition range of the mineral speciesis large, as
in augite, one or more adjectival modifiers are used to
specify the composition more clearly (e.9., subcalcicaugite, Fe-rich augite).
ries (adeite-aegirineseries).Subdivision namesof the intermediate solid-solution ranges, such as bronzite,
hypersthene,and eulite of the enstatite-ferrosilite series
and salite and ferrosalite ofthe diopside-hedenbergiteseries, have been discarded. However, the 500/orule was
not applied rigorously to the Ca-Mg-Fe pyroxenes and
Na-Ca pyroxenes.The widely acceptedterms such as augite, pigeonite,omphacite, and aegirine-augitee
have been
retained.
Gem namesof spodumene
Two names,"hiddenite" and "kunzite," are often used
for (pale)emerald-green-and lilac<olored spodumeneof
gem quality, respectively. They are not acceptedas formal pyroxene names, but can be used as varietal gem
names.
Relationshipswith the pyroxenoids
Pyroxenoids are closely related to pyroxenes in that
they have a similar type of chemical composition and a
structure that also consists of SiO, single chains. However, the repeat of the chains, which is two SiOo tetrahedra in the pyroxenes,is three or more SiOotetrahedra
in the pyroxenoids.Although the tetrahedral sitesin both
the pyroxenesand the pyroxenoids are mostly occupied
by Si ions, the large cations in the pyroxenoids are mostly
Ca, Mn, and Fe2*ions. The classificationand nomenclature ofthe pyroxenoids are beyond the scopeofthis report. However, the following two points may be noted.
First, there is a polymorphic relationship with some pyroxenessuch as ferrosilite, hedenbergite,and johannsenite. These show pyroxenoid structures at high temperatures or pressures. Second, the wollastonite chemical
component (CarSirOu)is used to expressthe composition
of the Ca-Mg-Fe pyroxenes,though wollastonite belongs
to the pyroxenoid structural group.
Cr,,lssrrrcauoN
AND NoMENCLATUREoF THE
PYROXENES
Preliminary classifications:Construction of the Q-"I
diagram and application of pyroxene data
Before classifoing the pyroxenes into the 20 mineral
specieslisted in Table 2, the following procedure is recommended to divide them into four chemicalgroups:Capyroxenes(Quad), Na-Ca pyroxenes(Na-Ca), Na
Mg-Fe
Application of 500/orule
pyroxenes(Na), and other pyroxenes(Others) (Morimoto
The 500/orule has been applied to complete solid-so1983).
lution seriesbetweentwo end membersas far as possible. and Kitamura,
procedure the pyroxenesare classifiedby using
In
this
They are the Mg-Fe pyroxene series(enstatite-ferrosilite
the total numbers of specifiedcations at the M (Ml and
and clinoenstatite-clinoferrosiliteseries),Ca pyroxene seM2) sites on the basis of six oxygens.The Ml and M2
ries (diopside-hedenbergiteseries)and Na pyroxene sesites are consideredtogether as M sites, without considering the site preferenceof atoms between the two sites.
7CarSirOu
existsas wollastonitein nature,which belongsnot
The numbers of Ca, Mg, Fe2*, and Na cations in the
To represent
to the pyroxenes
but to the pyroxenoids.
the comM
sites are plotted in the Q-,Idiagram (Fig. 2) as Q : Ca
positionsof the Ca-Mg-Fep)"roxenes,
(WoF
the ternaryCarSLOu
(Fs) systemhas beenused,e.g., + Mg + Fe2* and J : 2Na. The lines representingthe
MgrSirO6(En)-FerSirOu
EnroFsrrWoor.
t Definition of the "Other pyroxenes" is different from that
given by Cameronand Papike(1981).
eThe name "aegirine-augite" appearsto be in more common
usagethan "aegirineaugite," and "acmite-aug1te."
MORIMOTO:NOMENCLATUREOF PYROXENES
tr27
,ES
I
I
I
Je
J
Fig. 2. Q-J diagram for the pyroxenes,on which the posi
tions of the I 3 acceptedend members have been indicated. Abbreviations and cornpositions of the end members are listed in
Table 2.
Fig.3. The103,r.o*.rrlrouo- Deeret
tllzsl selected
by CameronandPapike(1981)plottedon the"r.
Q-./diagram.For
the O valuesarelessthan 1.90,and Mn is less
thesepyroxenes,
than 0.08atomsper formulaunit.
+ Mg + Fe (Quad), Ca-Na, and Na pyroxenes. The
boundariesdefinedbv J/(Q + J):0.2 and 0.8 are used
by Deer et al. (1978)and Cameronand Papike (1981).
following equations are used to subdivide the Q-J diaBecausethe Mn-Mg pyroxenesand johannsenite (Tagram:
ble 2) have Mn ions occupying more than half of the M2
( l ) and Ml sites, respectively, they have Q values between
Q + J:2.0
(2) 1.0 and 1.5 in the Q-J diagram. Similarly, petedunnite
Q+J:r.5
(3) and esseneiteplot along the Q axis with Q valuesbetween
J/(Q + J): 0.2
(4) 1.0 and 1.5. Spodumene plots at the origin of the Q-J
J/(Q +.I) : 0.8.
The areascorrespondingto the Ca-Mg-Fe pyroxenes,Ca- diagram becauseboth Qand J arezero. Thus, the thirteen
Na pyroxenes, Na pyroxenes, and other pyroxenes are end members (Table 2) and Wo are located in the Q-J
labeled (Fig. 2) Quad, Ca-Na, Na, and Others, respec- diagram (Fig. 2).
Application ofthis classificationprocedure to 406 pytively.
In this diagram, ,Iis meant to include the total number roxeneanalysespresentedin Deer et al. (1978) has shown
of Na and R3*, usually Al, Fe3*,Cr3*, and Sc3*,that cou- that most of the analyses,except those of johannsenite
ple with Na in substitution I mentioned in Table l. When and spodumene, are included in the area between the
the coupled substitution in the pyroxene is not oftype I lines O + J : 2.0 and Q * J : 1.5. The 103 Deer et al.
but of type 2 or 3, lhe J value apparently does not rep- (1978)pyroxenesselectedby Cameronand Papike (1981),
resent the real numbers of Na and R3* at the M sites. for which the Q values are less than 1.90 and Mn is less
However, substitution 3 (e.g.,Al-Al) works to move the than 0.08 atoms per formula units, are plotted inthe Q-J
J and, Q values closer to the origin of the Q-J diagram, diagram of Figure 3. The "CaMgTAL" pyroxene (Camand substitution 2 (e.g., Na-Tio') to move the ,I value eron and Papike, l98l) is included in the Quad area as
farther away from the Q axis ofordinates. Therefore, the described later in this report (Table 3, no. l). Only 20
effectsof substitutions 2 and 3 tend to cancel each other analyses among 406 plot slightly over the line Q + J :
out in and near the area of the Na pyroxenes.Thus the "/ 2.0, and most of these show unusual total numbers of
(: 2Na) values in the Na-rich pyroxenesrepresent,to a cations. The results ofthe classificationofthe pyroxenes
good approximation, the total number of Na and R3' (A1, into the four chemical groups by this procedure are in
Fe3*,Cr3*, and Sc3*)at the M sites.
almost complete agreementwith the results obtained by
Deer et al. (1978)and by Cameronand Papike(1981).A
The boundary Q + J: 2.0 representsthe upper limit
of Q + J ar the M sites. The boundary Q + J : 1.5 few unusual pyroxeneswith Mn less than 0.08 atoms for
representsthe limit below which more than half of the the chemical formula unit have been found to lie outside
Ml or M2 sites may be occupied by ions other than Q the areabetweenthe lines Q + J: 2.0 and Q + J : 1.5
and ,I ions. In this case,the pyroxenesare consideredas n the Q-J diagram. The classification of these unusual
belonging to Others, which include the Mn-Mg and Li pyroxeneswill be discussedlater in this report.
pyroxenes,johannsenite, petedunnite, and esseneite.
The pyroxenesthat plot in the area between Q + J:
Equations 3 and 4 represent the lines dividing the area 2.0 and Q + J : 1.5 have componentsthan Qand,Iions
limited by the two above-mentioned Q + "Ilines into Ca at lessthan 25o/oof the M sites.Therefore.we can classify
I 128
MORIMOTO:NOMENCLATUREOF PYROXENES
TABLE
3. Chemical
composition
andclassification
of eightunusualpyroxenes
A. Ca-rich group related to S3 and S4
Si
AI
AI
Ti.*
Ti0*
Fe3*
Mg
Fee+
Mn
Na
K
1: 320-8
(406-16)
2:403-3
1.443
2.00
o.577
0.091
0.165
1.506
2.00
0.494
0.'t71
0.065
1.434
2.00
0.566
0.306
0.o22
0.128
0.385
0.2292.00
0.005
0.992
0.006
0.000
0.159
0.570
0.0632.02
0.007
0.975
0.007
0.001
0.218
0.408
0.0602.00
0.005
0.979
0.002
1.61
0.01
1.61
0.01
1.45
0.00
3: D andS-
B. Na-richgroup related to S2
4: T andR-1. 1 9 6
2.00
0.804
0.186
0.111
0.394
0.289
2.00
1.021
1.31
0.00
5: 488-9
7:492-19
C andGf
6:491-14
8: C andG{
1.994
2.00
0.032
0.000
0.265
2.024
2.02
0.000
0.021
0.023
2.026
2.03
0.000
0.098
0.227
2.01
0.000
0.348
0.104
0.458
0.150
0.1072.00
0.003
0.083
0.933
o.728
0.070
0 . 11 3 2 . 0 0
0.006
0.155
0.872
0.009
0.34
1.74
0.192
0.070
0.4201.98
0.021
0.152
0.794
0.031
0.168
0.3562.00
0.011
0.361
0.610
0.006
0.89
1.22
0.34
'1.87
Mineral
names
subsilicictitanian
ferrian diopside
subsilicic
aluminian
ferrian
diopside
subsilicic
aluminian
ferrian
diopside
subsilicictitanoan
aluminian
pyroxene
titanian
magnesran
ferroan aegtnne
Namesin
literature
titanaugite(3208)
titanium fassaite
(406_1
6)
CaM9TAL(C
and P)t
fassaite
fassaite
titanaugite
titanianaegir- aegirineine
augite
calcian ferroan aegirine
0.64
1.59
titanian aegirineaugite
titanianferroanomphacite
titanian aegirineaugite (49219)
titanian aegirine
(c and G)t
titanianferroomphacite
Nofe.'Numbers such as 320-8, etc. represent pages and analysis number in Deer et al. (1978).Other referencesare in text. With the exception of
320-8 (:406-16), all the Deer et al. (1978)analysesin this table were not includedin the 103 selectedanalysesof Cameronand Papike(1981).Atl
pyroxenes in the table are shown with their numbers in the O-Jdiagram (Fig. 7). S2, 53, and S4 represent the following components of substitutions
2, 3, and 4, respectively:52 : NaRAjTi6.tSi,O.,S3 : CaR3*AlSiOu,
and 54 : CaRAITiS.gAlSiOu.
To indicate R ions explicitly in these components,the
notation S(R), such as S2(Mg) and S3(AD,is used. S3(Fe)is a new pyroxene, esseneite(Es). Component ratios for the eight samples are as tottows:
( ' 1 ) l W g i e l ' : f q ' o ) n o s 4 ( M g ) " s 4 ( F e ) 1 6 E s 1 3 S 3 ( A l()2, ,) ( w o . u E n , 5 F s r ) 5 s S 3 ( A l ) r 7 E s 1 6 S 4 ( M g ) , , s 4 ( 3F)e (Lw, o . . E n . o F s . ) . . S 3 ( A t ) . , E s , , S 4 ( M g ) 0 ,
(j) S3(TD""S4(Mo).r(wo',Fsr)aS3(Al),.,(5) AeouS2(Mg)".S2(Fe),0^6,
(6) (AeTaJd,wo6Fs6Eni*S2.a., (7) (Ae,eJd,oFs,,wo"En,)r,S2(Fe)*s2(l\4g).a",
(8) (Jd..Ae"Wo'.Fs15En7D6S2(Fe)1rS2(Mg)r4..
The symbol a representsminor components,some of which have unusuafmetal Aiios ior ini pyroiene
structure.
* Devineand Sigurdsson(1980),
Table 1 for fassaite.
't Tracy and Robinson(1977),
Table 3, analysis I for pyroxene from the Allende meteorite(Mason, 1974).
t Cameronand Papike(1981),TabteA3, anatysis920-8 and 406-16.
+ Curtisand Gittins(1979),Table 2, analysis5 for no. 7 and TabteS, anatysis5 for no. 8.
such pyroxenes on the basis of the normalized Q and J
components, thereby neglecting the effects of the other
components. The following procedures are adopted for
further classification:(l) The pyroxenesin the Quad area
are classified on the pyroxene quadrilateral Wo-En-Fs
diagram with normalized Ca, Mg, and >Fe (: psz++ Fe3*
+ Mn) atoms. (2) The pyroxenesin the Na area are jadeite, aegirine, kosmochlor, and jervisite. Becausekosmochlor and jervisite show little or no solid solution toward other end members, they play no role in the
classification.Jadeite and aegirine are classified on the
Quad-Jd-Ae diagram togetherwith the Ca-Na pyroxenes,
aegirine-augiteand omphacite.
Classificationof the Ca-Mg-Fe 66quadrilateral"pyroxenes
The common rock-forming pyroxenesform wide ranges
of solid solutions of the Ca-Mg-Fe pyroxenesand can be
expressedby the pyroxene quadrilateral of the MgrSirO.
(EnlFel-SirOu @spaMgsiro6 (DiFCaFer*SirO6 (Hd)
system. The Ca-Mg-Fe pyroxenesinclude varieties that
have orthorhombic symmetry. These orthopyroxenes
consist essentially of a simple chemical series
(Mg,Fe)rSirO6and thus contrast with the Ca-Mg-Fe cli-
nopyroxenes,which have wide rangesof chemical composition. Therefore, the Ca-Mg-Fe pyroxenesare defined
on the basisof symmetry and relative amountsof CarSirO.
(Wo), MgrSirOu(En), and Fe!*SirO. (Fs). The composition rangesofthe clinopyroxenesand orthopyroxenesare
indicated in Figures4 and 5, respectively,where the composition is normalized to Ca * Mg + ZFe : 100 with
)pg : pgz++ Fe3*+ Mn2+.r0
r0For the nomenclature of the Ca-Mg-Fe pyroxenes,normalization must be made to Ca * Mg + ZFe : 100, where )Fe :
Fe2* * Fe3++ Mn. Hereafter the mole percentof the end member components is always used without remark and is representedsimply by o/0.If the mole percentsof quadrilateral components are calculatedby the atomic percent ofCa to the total
cations at the M sites, no pyroxenesshould contain more than
50o/oCarSizOu.However, if Ca, Mg, and Fe are normalized, or
+ Mg + >Fe), 100 Me/(Ca + Mg +
calculatedas 100Ca,u(Ca
)Fe), and 100 ZFel(Ca + Mg + )Fe), respectively,then some
augites will plot on a Wo-En-Fs triangular diagram above the
50o/oCa,Si,Ouline. Especiallywhen the plot in the Q-J diagnm
is very close to or outside of the boundary Q + t : 1.5, the
effectofjohannsenite and petedunnitecomponentsmust be considered.Ifthe effect is negligible,the pyroxene must be considered to have an unusual composition and must be referred to
the section ofunusual pyroxenes.
rr29
MORIMOTO:NOMENCLATUREOF PYROXENES
Co rSirO,( Wo)
diopside
\
lhedenbergite
\
c2/ c
ou9ite
\
pigeonite
clinoenstotite
clinoferrositite
I
50
M92Si205(En)
P 27/c
\
\
F e r S i r O 5( F s )
Fig. 4. Composition rangesof the Ca-Mg-Fe clinopyroxeneswith acceptednames.
The distinction betweenaugiteand pigeonite in the CaMg-Fe pyroxenesis primarily structural, their spacegroups
being C2/c and.P2,/c, respectively.There is a miscibility
gap between augite and pigeonite, and many pyroxenes
with I 5-250/oWo have proved to be mixtures of the two.
Augite with less than about 250loWo is often called subcalcic augite.On heating,pigeoniteundergoesa rapid displacive transformationlo a C2/c structure, which cannot
be quenched.Augite does not show this type oftransformation.
The most Ca-rich orthopyroxene contains approximately 50/oWo. The high-temperature form of enstatite
has the spacegroup Pbcn and can be expressedas "enstatite-Pbcn." This form is not quenchableand has not
been found in nature. "Protoenstatite" has been used
conventionally to describethis form, but this name is not
adopted as a mineral name. The Wo value of "enstatitePbcn" doesnot exceed2o/o,and the En value commonly
exceeds900/0.Thus the composition field of "enstatitePbcn" is different from that of enstatite-Pbca.
jervisite, these two speciesare separatelytreated in the
classification of the Na pyroxenes.Both jadeite and aegirine, however, show extensive solid solution with the
Ca-Mg-Fe pyroxenes, especially with the diopside-hedenbergiteseriesand augite, leading to the Ca-Na pyroxenes.The Na and Ca-Na pyroxenesare classifiedon the
Quad-Jd-Ae diagram (Fie. 6) with normalized Q (Wo +
En * Fs), Jd, and Ae components." The arbitrary divittTo normalize
Q, Jd, and Ae components,the sum of Ca +
Mg + Fe2* + 2Na at the M sites must be made to total 100%.
Then the normalized percentageof 2Na must be divided into
the ratio of AVFe3* to give the ratio of Jd/Ae. Thus Q + Jd +
Ae must always give 1000/0.
When the plot in the Q-J diagram
is significantly outside the boundary Q + J :2.0, the effect of
substitution 2 must be considered,as in the section ofunusual
pyroxenes.
Q (Wo,En,Fs)
/"
Classification of the Na and Ca-Na pyroxenes
The Na pyroxenes, jadeite and aegirine, commonly
contain more than 900/oof the NaAlSirO6 or NaFe3*Si2O6
component, respectively,but contain neither the Ko nor
the Je component. Becausekosmochlor is a rare accessory constituent of some iron meteorites and only one
terrestrial locality is known for each of kosmochlor and
omphocite
"\
o€irine-q€ite
qegirine
iodeite
MgzSi?06(En)
Fe?Si,O6(Fs)
Fig. 5. Composition rangesoforthopyroxenes with accepted
names.
NoAlSi2 Og (Jd )
50
\
NoFe3'si2o5(Ae)
Fig. 6. Ca-Mg-Fe and Na pyroxenes with acceptednames.
Quad represents the Ca-Mg-Fe pyroxene area (see Fig. 4).
MORIMOTO:NOMENCLATUREOF PYROXENES
l 130
Tnaue5. List of adjectivalmodifiersto be usedfor pyroxene
mineralnames
Content"
Al3+
Ca2*
Cre*
Fe2*
Fe"'
Li*
Mg*
Mn,*
Fig. 7. Q-l diagramfor eight unusualpyroxeneswith Q value
less than 1.62 and Mn less than 0.08 atoms per formula unit
(Table 3). The components formed by the substitutions I to 4,
as indicated in Table l, are plotted in the diagram. They represent the following compositions: Sl : NaR3*SirO6, 52 :
NaRfrjTiijSi,O", 53 : CaR3*AlSiOu,and 54 : CaRijTiSjAl-
sio,.
sions between the Ca-Mg-Fe pyroxenes, Na-Ca pyroxenes,and Na pyroxenesare defined at 20o/oand 80o/oof
0. Omphacite displays a C2/c = P2/n polymorphic transition, and both high-temperatureC2/c and low-temperatureP2/n polymorphs appear in nature. Omphacite can
thus be divided into two subspecies:omphacite-C2/c and
omphacite-P2/n. Becauseomphacite-P2/n shows a unique
crystal structure different from that ofjadeite and augite,
it is acceptedas an independentpyroxene species.Aegirine-augite is also acceptedas an independent speciesto
keep balance with omphacite, though it is not known to
Mns+
Nat
Ni2+
si.*
Ti3*
Ti4*
Zn2+
>0.10
>0.10
>0.01
>0.10
>0.10
>0.01
>0.10
>0.10
>0.01
>0.10
>0.01
<1.75
>0.01
>0.10
>0.01
Name
aluminian
calcian
chromian
ferroan
ferrian
lithian
magnesran
manganoan
manganran
sodian
nickeloan
subsilicic
titanoan
titanian
zrncran
Note.'The limit of the content is based on the values listed in Table 4.
* Numberof cations per formulaunit M2M1TrO6.lf the mineralname
itself impliesthe presenceot certain cations, adiectivalmodifiersfor these
cations should not be used ("subsilicic" is an exception).
occur with the P2/n structwe. The classification of the
Ca-Na pyroxenesby Esseneand Fyfe (1967) is not followed in this report.
Classification of other pyroxenes
Most naturally occurring pyroxenes in the Others area
are johannsenite (CaMnSirO.), petedunnite (CaZnSirOr),
and spodumene (LiAlSirOu) (Fig. 2). Recent investigations of natural Mn-bearing pyroxeneshave yielded two
new minerals, kanoite and its dimorph donpeacorite,
TneLe4. Extremechemicalcompositions
of pyroxenesin Deer (Mn,Mg)MgSirOu, which seem to form a solid solution
et al.(1978)
with En (Peterson et al., 1984). They too occur in the
Mg-Fe
Others area. These results suggesta possible Mn-Mg-Fe
pyroxenes
Na pyroxenes
Ca pyroxenes
pyroxene quadrilateral. Esseneite(CaFe3*AlSiOu)is the
1.94(488-9)
first pyroxene with the substitution 3 as describedin Ta1.76(42-91
1.44 (320-8r
Si
0.07(488-8)
A13*
o.24(42-9)
0.56 (320-8)
ble l.
Fes*
Al3*
Ti1*
Fe3+
Mgz*
Fe'?*
Mn2*
Ct2*
Ni2*
Zn2+
Ca2*
Na*
0.04(49-8)
0.15(49-6)
0.04(40-30)
0.12(170-8)
1.99(41-1)
't.72(47-33)E
0.27(45-21)F
0.02(36-9)
0.26(169-2)
0.10 (169-2)
0.09 (320-11)
0.35 (320-11)
0.17 (320-8)8
0.37 (321-5)D
1.27 (208-4)
1.09 (220-13)
0.36 (217-5)G
0.06 (207-11)
0.003(317-1)
0.21 (216-11)'
1.O3 (202-4l.
0.31 (323-7)
0.02(488-9)
0.98(464-1
)
0.27(488-9)c
0.s7(487-1)
0.15 (488-9)
0.11(488-9)
0.03(487-4)
4)
0.16 (466-1
0.98(464-1)
Note.'Givenare the numberof cationsoer tormulaunit.minimumvalues
for Si, and maximum values for other cations. Bold numbers are for the
mainconstituentelements.Numbersin the oarenthesessuch as 42-9, etc.,
indicatepages and analysisnumbersin Deeret al. (1978).Other references
are in text.
a Table 3, no. 1; Table 3, no. 4: Pyroxene from the Allende meteorite
1.20 (Mason,1974; Tracy and Robinson,1977).
6 Probe analyses 0.252 and 0.282, halt ol CaRAITidjAlSiO6(S4) Crracy
and Robinson,1977; Robinson,1980).
c Table3, no. 5. Half of NaR6:Ti63SirO6
(S2).
D406-150.67,omittedbecauseof possibleerrorsin chemicalanalysis.
EProbeanalysis1.880(Jaffeet al., 1978).
FProbeanalysis0.301(Robinson,1980),kanoite 1.04 (Kobayashi,1977).
c Johannsenite0.963(417-2).
HKosmochlor0.90 (522-1).
'Petedunnite 0.37 (Table 2, footnote with asterisk).
Classification of unusual pyroxenes
Severalpyroxeneswith unusual chemical compositions
(Table 3) appear outside the area between the lines Q +
J : 2.0 and Q + J : 1.5 in the Q-,Idiagram, though they
do not belong in the area of Other pyroxenesmentioned
above (Fig. 7). They contain large amounts of chemical
components involved in substitutions 2, 3, and 4 mentioned in Table l.
These pyroxenescan be divided into two groups: first,
Ca-rich pyroxenes with CaR3*AlSiO6(S3, Fig. 7) and
CaRtlTifi.lAlsio6 (S4, Fig. 7) components representing
substitutions 3 and 4, respectively,and second,Na-rich
pyroxenes with the NaKjTi6jSirO. component representing substitution 2 (52, Fig. 7). The former shows a
significant deficiencyof Si atoms such as Si < 1.60 in the
standard formula resulting in the O value closeto or less
than 1.5 (S4, Fig. 7). The latter appearsoutside the line
Q + J : 2.0 approachingpoint 52 in Figure 7. All these
unusual pyroxenes are classified by using the accepted
pyroxene names and the adjectival modifiers mentioned
I l3l
MORIMOTO:NOMENCLATUREOF PYROXENES
TraLe6. Obsoletepyroxenenames
Teew d-Continued
acmite: aegirine
aegirite(aegyrite): aegirine
aegerine-hedenberyile: augite
agalite : probably enstatitepaftly altered to talc
agfaite = alteted spoclumene
alalite: diopside
afkali augite : aegirine-augite
ambfystegite: enstatite
anthochroite: augite
asteroite : iron-rich(or Fe-rich)augife
baikalite : diopside
bastite : enstatite that has altered to serpentine,talc, or perhaps anthophyllite
blanfordite: manganoanaegirine-augite
bronzite: enstatite
calc-cfinobronzite: pigeonite
calc-cfinoenstatite: pigeonite
calc-cfinohypersthene: pigeonite
calc-pigeonite= subcalcic auglte
canaanite: diopside
chfadnite: enstatite
chforomelanite: omphaciteor aegirine-augite
chrome-acmite : chromian aegirine
chromejadeite : chromia jadeite
clinohypersthene : clinoenstatite ot clinoferrosilite
coccolite (kokkolith) : iron-rich(or Fe-rich)augite
cymatofite: altered spodumene
diaclasite: altered enstatite
diallage = diopsidelhal has altered or that has good (100) parting; also
used tor alteration products of other pyroxenes
diopsidjadeite: omphacite
endiopside: magnesium-rich(or Mg-rich) augite
enstatite-diopside: magnesium-rich(or Mg-rich) augite
eulite: ferrosilite
eulysite : ferrosilite
fassaite : ferrian aluminiandiopsideor augite
fedorovite = diopside
ferroaugite: augite
ferrohedenbergite: augite
ferrohypersthene: fenosilite
ferro-iohannsenite: iron-rich(or Fe-richljohannsenite
ferropigeonite: iron-rich(or Fe-rich)pigeonite
ferrosalite : hedenbergite
ficinite: enstatite
funkite = hedenbergite
germarite : altered enstatite
hiddenite: spodumene
hudsonite : hedenbergite
hypersthene = enstatite ot terrosilite
jadeite-aegirine0adeite-aegirite): jadeite ot aegirine
jeffersonite: zincian manganoan diopsideor augite
killinite: alleted spodumene
korea-augite: augite
kunzite: spodumene
favroftite : diopside
favrovite = diopside
fawrowite = diopside
feucaugite: diopside
lime-bronzite : pyobably pqeonite or enstatite plus augite ("inverted"
pigeonite)
loganite: diopside + actinolite + talc
lotalite = hedenbergite
mafacolite: diopside with good (001) parting, also diopside from Sala,
Sweden
mansjoite : augiteot diopsideot hedenbergite
mayaite: omphacite
mellcrite: ofthopyroxene
mondradite: probably an altered pyroxene
mussite: d,psde
orthobronzite= enstatite
orthoenstatite : enstatite
orthoeulite : ferrosilite
orthoferrosilite : ferrosilite
orthohypersthene = enstatite ot ferrosilite
paufite = enstatite
peckhamite: enstatite
phastine : altered enstatite
picrophyff= altered pyroxendl
pigeonite-augite: probably subcalcicaugife
pitkarantite : pyroxene?
potash-aegirine: synthetic product, probably not properly characterized
protheite : augite
protobastite : enstatite
pyraffolite: altered pyroxene?, talc?
pyrgom: pytoxene
sahfite = diopside
salite : diopsde
schefferite: manganoandiopside
schillerspar(schillerspat): enstafite that is altered to serpentine,talc, or
anthophyllite
shepardite: enstatite
soda-spodumene: sodian spodumene
strakonitzite : altered pyroxene, steatite?
szaboite : partly altered enstatite
titanaugite : titanian augite
titandiopside: titanian diopside
titanpigeonite= titanian pigeonite
trachyaugite: augite
traversellite: dioDside
triphane: spodumene
tuxtlite = omphacite
uralite: pseudomorphof amphiboleafter pyroxenes
urbanite : iron-rich (or Fe-rich) augite ot aegirine-augite
ureyite: kosmochlor
vanadinaugite: vanadium-bearing(or V-bearing)augife
vanadinbronzite: vanadium-bearing(or V-bearing)enstatite
vargasite = altered pyroxene?
victorite: enstatite
violaite: augite
viofan : magnesium-rich(or Mg+ichl augite or dipside
/VotejThe above pyroxene mineralnames,or namesthat refer to altered
pyroxenes, have been formally discarded by the CNMMN. The correct
names are italicized.The original form of this table was compiled by Malcolm Ross usingthe followingreferences:Dana(1892); Tschermak(1897);
Chester(1886); Ford (1932);Winchelland Winchell(1951); Deer et al.
(1963,1978);Strunz(1970);and the unpublishedThesaurusof MineralogicalTermsof the InternationalMineralogicalAssociation,which has been
availablesinceAugust 1974.
below, exceptthe Allende pyroxene(Table 3, no. 4), which
is called subsilicic titanoan aluminian pyroxene.
of the S3(Ti)
The Allende pyroxene(no. 4) contains390/o
(for notation, see note in Table 3) component
CaR3*AlSiO6,where R3+: Ti, and can be consideredas
a new mineral. However, we have decided only to use
the acceptednamesin this report and ifa specieshas not
yet been approved, w€ use "pyroxene" as for no. 4 in
Table 3. The names used in literature for the unusual
pyroxenesare listed in Table 3 in comparison with those
in this report. The "CaMgTAL" pyroxene (no. l) is
"diopside" in this classification.
Anrucrrvll
MoDTFTERS
Adjectival modifiers for mineral names are used to indicate unusual amounts of chemical constituents. In order to define the unusual amounts for the pyroxene mineral group quantitatively, extreme compositions of
pyroxeneshave been listed in Table 4, where the values
for the main cations are shown as well as those for the
accessoryqrtions. Deeret al. (1978)and Robinson's(1980)
table were mainly used in constructing Table 4.
An element specifiedas a modifier should be present
as a generalrule in a quantity larger than 0.1 atoms (or
rr32
MORIMOTO:NOMENCLATUREOF PYROXENES
0.01 for lessabundant elements)in the standardchemical
formula of 6 oxygensor 4 metal atoms (Table 5) depending on the maximum content in Table 4.
The sufrxes are those proposedby Schaller(1930) and
adaptedby GNMMN (Nickel and Mandarino, 1987).The
suffix "-ian" is used for the higher valence state (e.9.,
ferrian) or for an element with a nonvariable state (e.g.,
lithian). The suffix "-oan" implies the lower valencestate
(e.g., ferroan). It is recommended that such modifiers
never be used for main cations normally contained in the
named mineral, for example, in terms like "calcian augite," "aluminian omphacite," and "sodian aegirine-augite," in which the modifiers are obviously superfluous.
If there is less than the amount necessaryfor the assignment of the modifiers such as "aluminian" in Table
5, or <0.1 Al, but if the increasedcontent of the element
must be stressed,a modifier "Al-bearing" may be used.
This second type of modifier should be used also (l) if
only an incomplete analysis is available, preventing the
calculation of a full chemical formula, or (2) for pyroxeneswhere the valencestateof a cation is unknown. With
regard to the Si content in pyroxenes,it is suggestedthat
Si < 1.75 is a suitable limit for use of the term "subsilicic," though one should bear in mind that the limit of Si
< 5.75 for "subsilicic" in amphiboles correspondsto Si
< 1.5 for pyroxenes.
In certain cases,particularly for the augite series,it is
convenient to use the following adjectival modifiers: Ferich, Mg-rich, and subcalcic. A prefix actually attached
or hyphenated to a mineral name, however, is incorrect
and should be avoided (Nickel and Mandarino, 1987),
becauseit would causethe mineral to be indexed alphabetically under the prefix rather than under the proper
mineral name. This is why such terms as "ferropigeonite," "ferro-augite," etc., should not be used as mineral
names.
It is often useful to give the spacegroup of the mineral,
particularly when it can occur in two or more forms. For
example, we could distinguish between the two forms of
omphacite by adding the space-groupsymbol., i.e., omphacite-C2/c vs. omphacite-P2/n, or by adding the lattice-type symbol, i.e., omphacite-C vs. omphacite-P (Bailey, 1977).
Onsolrrn
PYRoxENENAMES
The namesof 105 pyroxenesor alteredpyroxeneslisted
in Table 6 have formally been discardedby the CNMMN
and are therefore obsolete. The preferred name is italicized in the same table.
AcxNowr-rncMENTs
We are thankful to ProfessorJ.H.D. Donnay, McGill University, Montreal, who contributed greatly to the improvement of the report by his
careful review. We also appreciatecriticisms and comments by Dr. A.
Kato, National ScienceMuseum, Tokyo, Dr. M. Kitamura, Kyoto University, Kyoto, and the memben of the Commission on New Minerals
and Mineral Names, IMA.
Rrrnnnucrs crrED
Bailey, S.W. (1977) Report of the IMA-IUCr joint committee on nomenclature. American Mineralogist, 62, 41 1415.
Bokij, G.B., and Ginzburg, J.V. (1985)The systematicsof mineral species
in pyroxenefamily. Trudy Instituta Geologii i Geofiziki (Novosibirsk),
610, 12-35.
Cameron, M. and Papike, J.J. (1981) Structural and chemical variations
in pyroxenes.American Mineralogist, 66, 1-50.
Chester,A.H. (1886) Catalogueof minerals. John Wiley and Sons,N.Y.
Cosca,M.A., and Peacor,D.R. (1987) Chemistry and structureof esseneite (CaFe3*AlSiOu),
a new pyroxene produced by pyrometamorphism.
American Mineralogist, 72, 148-156.
Curtis, L.W., and Gittins, l. (1979) Aluminous and titaniferous clinopyroxenesfrom regionally metamorphosedagpaitic rocks in central Labrador.JournalofPetrology,20, 165-186.
Dana, E.S. (1892) The system of mineralogy (6th edition). Wiley, New
York.
Deer, W.A., Howie, R.A., and Zussman, J. (1963) Rock-forming minerals,vol. 2, Single-chainsilicates(first edition). I-ongman, Green and
Co. Ltd., London.
(1978) Rock-forming minerals,vol. 2A, Single-chainsilicates(2nd
edition). Longman, U.K. and Wiley, New York.
DeVine, J.D., and Sigurdsson,H. (1980)Gamet-fassaitecalc-silicatenodule from La Soufriere,St. Vincent. American Mineralogist, 65,302305.
Dowty, E. and Clark, J.R. (1973) Crystal structurerefinementand optical
propertiesof a'Ti3* fassaitefrom the Allende meteorite.American Mineralogist, 58, 230-240.
Essene,E.J.,and Fyfe, W.S. (1967) Omphacite in California metamorphic
rocks. Contributions to Mineralogy and Petrology,15, l-23.
Essene,E.J., and Peacor, D.R. (1987) Petedunnite (CaZnSirOu),a new
zinc clinopyroxenefrom Franklin, New Jersey,and phaseequilibria for
zincian plroxenes. American Mineralogist, 72, 157-166.
Ford, W.E. (1932) A textbook of mineralogy. Wiley, New York.
Jaffe,H.W., Jaffe,E.8., and Tracy, R.J. (1978) Orthoferrosilite and other
iron-rich pyroxenesin microperthite gneissof the Mount Marcy area,
Adirondack Mountains. American Mineralogist, 63, I l6-136.
Kobayashi, H. (1977)Kanoite, (Mn'z.Mg),[Si,Ou],a new clinoplnoxenein
the metamorphic rock from Tatehira, Oshima Peninsula, Hokkaido,
Japan.Journal ofthe Geological SocietyofJapan, 83,537-542.
I-eake, B.E., and Winchell, H. (1978) Nomenclature of amphiboles.
American Mineralogist, 63, 1023-1052.
Mason, B. (1974) Aluminum-titanium-rich pyroxenes,with special reference to the Allende meteorite. American Mineralogist, 59, 11981202.
Mellini, M., Merlino, S., Orlandi, P., and Rinaldi, R. (1982) Cascadite
andjervisite, two new scandiumsilicatesfrom Baveno,Italy. American
Mineralogist, 67, 599-603.
Morimoto, N., and Kitamura, M. (1983) Q-J diagram for classification
of pyroxenes.Journal of the JapaneseAssociation of Mineralogists,
Petrologistsand Economic Geologists,78, 141 (in Japanese).
Nickel, E.H., and Mandarino, J.A. (1987) Proceduresinvolving the IMA
Commission on New Minerals and Mineral Names and guidelineson
mineral nomenclature.CanadianMineralogist, 25, 353-377; American
Mineralogist, 72, 103l-1042.
Papike,J.J.,Ed. (1969)Pyroxenesand amphiboles:Crystal chemistry and
phasepetrology. Mineralogical Society ofAmerica SpecialPapet 2.
Peterson, E.U., Anovitz, L.M., and Essene,E.J (1984) Donpeacorite,
(Mn,Mg)MgSLOr, a n€w orthopyroxene and its proposed phase relations in the system MnSiOj-MgSiOr-FeSiOr. American Mineralogist,
69,472480.
Prewitt, C.T., Ed. (1980) Reviewsin mineralogy,vol. 7. Pyroxenes.MineralogicalSocietyof America, Washington,D.C.
Robinson, P. (1980) The composition space of terrestrial pyroxenesInternal and externallimits. Mineralogical Societyof America Reviews
in Mineralogy, 7, 419494.
Schaller, W.T. (1930) Adjectival endings of chemical elements used as
modifiers to mineral names.American Mineralogist, 15,566-574.
MORIMOTO: NOMENCLATUREOF PYROXENES
Strunz, H. (1970) MineralogischeTabellen, 5 Auflage.AkademischeVerlagsgesellschaft
Geest and Portig K.-G., Leipzig.
Tracy, R.J., and Robinson, P. (1977)Znnallitanian augitein alkali olivine
basalt from Tahiti and the nature of titanium substitutions in augite.
American Mineralogist, 62, 634-645,
Tschermak,G. (1897) khrbuch der Mineralogie. Alfred Holder, Wien.
Vieten,K.andHamm,H.M.(1978)Additionalnotes"Onthecalculation
of the crystal chemical formula of clinopyroxenes and their contents of
l 133
Fen*from microprobe analyses.Neues Jahrbuch .fiir Mineralogie Monarshefte,71-83.
Winchell, A.N., and Winchell, H. (1951) Elementsof optical mineralogy.
Wiley, New York.
MeruscnnrREcErvEDMencsT, 1988
MAr*uscx.rpr lccsprED Mav 6, 1988