Homework 14-15 c Silicates
advertisement

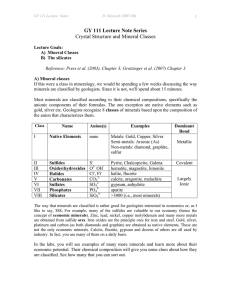
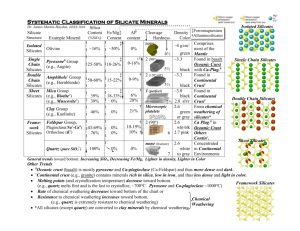
Homeworks 14 and 15 Silicates I and II Name _____________________ A. Lecture on Silicates I Part 1 Follow along in the PowerPoint and answer the following: 1. Explain why Silicates are the most abundant minerals. 2. Calculate the charge of the SiO4 anionic complex. 3. List the silicate types in order of crystallization from hottest to coolest from a basaltic melt. 4. Explain how Silicates polymerize. 5. Explain why silicate ion tetrahedra don’t share faces or edges. 6. Which has a stronger Al – 0 bond, (choose: Al+3 in tetrahedral coordination / Al+3 in octahedral coordination). Hint: check Electrovalency. 7. Name the biggest and smallest ions. Be sure to show their charge. Part 2 Match the Terms 8. Single chain Inosilicates ______ a. Olivines 9. Framework - Tectosilicates _____ b. Micas 10.Sheets - Phyllosilicates ______ c. Feldspars 11.Double Chain Inosilicates ____ d. Amphiboles 12.Independent Tetrahedra ____ e. Pyroxenes Part 3 Continue along in the PowerPoint and answer the following: 13. What is the generalized anionic formula for a Nesosilicate? 14.Why does Olivine have two metals per silicate ionic complex, (Mg,Fe)2SiO4 , but Zircon ZrSiO4. has only one metal per silicate ionic complex? 15. The generalized formula for Garnets is A3B2Si3O12 The B position in Garnet is usually Aluminum ion Al+3 . The Aluminum is (choose: in tetrahedral coordination / in octahedral coordination). 16. Look at the phase diagram for Al2SiO5. a. How many components? b. How many phases? c. Which phase has the lowest Gibbs Free Energy for P = 5 kilobars and T = 875oC? 17.What is the hardness of Topaz? 18.Describe the appearance of Staurolite in a Muscovite Schist. 19.In Epidote, Aluminum ion Al+3 is in octahedral coordination [6] with six oxygens OR four oxygens plus two ___________ ions. 20.The sorosilicate Hemimorphite, Zn4(Si2O7)(OH)2.H2O most frequently occurs as the product of the oxidation of the upper parts of Sphalerite ZnS bearing ore bodies, accompanied by other secondary minerals which form the so-called iron cap or ___________. B. Lecture on Silicates II 1. Tourmaline contains Boron. True or False? 2. Name three (3) Clinopyroxenes. 3. Name two (2) Orthopyroxenes. (Hint: Pigeonite is monoclinic). 4. Clinopyroxenes belong to the Orthorhombic Crystal System. True or False? 5. There is complete Mg-Fe solid solution between Diopside and Hedenbergite. True or False? 6. Pigeonite, a Clinopyroxene, is only stable at higher temperatures and exsolves to Augite plus an Orthopyroxene if cooled slowly to lower temperatures. True or False? 7. Two cleavages at about 90o are visible in pyroxenes when looking down the (choose: a – axis / b-axis / c – axis). 8. In Amphiboles, the largest Ions (Na+ or K+ ) sit between the double chains of silicate tetrahedra. True or False? 9. In the pyroxenoid Wollastonite, CaSiO3 , the orientation of silicate tetrahedra repeats every (choose: third / fifth / seventh) tetrahedron. 10. Explain why all the octahedral sites in Phlogopite have magnesium ions, but in Muscovite two of every three octahedral sites are vacant. 11. Examine the Phase Diagram for SiO2 a. How many components? _____ b. How many phases can you find? ____ c. Which phase has the highest entropy?____________ 12. Consider the pyroxene Enstatite (MgSiO3). The Gibbs Free Energy of Formation for Enstatite from pure elements (Mg, Si and O) = ΔGf (Enstatite, elements) is about -1,460.9 Joules*/mole at room temperature and pressure. Since the ΔGf values for Enstatite is negative, this means that Enstatite is more stable than, and will form from, the separate elements. a. True b. False * BTW: a Joule is a unit of energy, it has units Watts x seconds http://www.kean.edu/~csmart/Mineralogy/Lectures/Gibbs%20Free%20Energy%20of%20Formation.pdf