Grignard Reaction Preparation of Triphenylmethanol
advertisement

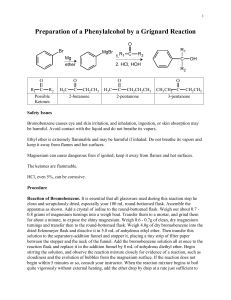
CHM!2202! Organic!Chemistry!Lab!II! Spring!2010! ! Department!of!Chemistry! Villanova!University! Grignard!Reaction! Preparation!of!Triphenylmethanol! From!Bell,!Clark!and!Taber,!pages!197!–!204! ! ! !!!!!!!!!!!!!! ! Perform!reaction!and!workup!in!hood.!!Ether!is!extremely!flammable!so!keep!away!from!flames!or!sparks.!! Make!sure!your!glassware!apparatus!is!absolutely!dry;!do!not!wash!your!reaction!flask!or!other!glassware! with!water!prior!to!carrying!out!this!reaction.! ! 1. Place!about!200!"!300!mL!of!water!in!a!400!mL!beaker!and!warm!the!water!to!~!60!–!65°C!by!heating! on!a!steam!bath!or!hot!plate.!!Do!not!allow!the!water!temperature!to!go!higher!than!65°C.! 2. Except! for! attaching! the! 10! mL! round! bottom! flask,! assemble! the! apparatus! depicted! in! the! accompanying!diagram.!!Examine!the!apparatus!set!up!on!the!cart! in! Mendel! 385! to! see! how! the! drying! tube! is! modified! to! accommodate!the!style!of!drying!tube!that!you!have.!!The!drying! tube!is!prepared!by!inserting!a!cotton!plug!at!the!bottom!of!the! tube,!filling!the!tube!with!drying!agent!(Drierite)!and!plugging!the! top!end!with!a!one"hole!rubber!stopper!or!a!plug!of!cotton.!!Put!a! very!very!thin!film!of!silicone!grease!on!the!joint!which!will!go!into! your!round!bottom!flask.!!Too!much!grease!will!contaminate!your! product!during!workup.! 3. Add!the!following!to!the!10!mL!round!bottom!flask!in!the!order! listed! 10 a. A!magnetic!stirring!bar! b. 0.16!mL!of!methyl!benzoate.!!Use!a!syringe!to!dispense! into!the!flask.! c. ~2.5!mL!of!anhydrous!ether.!!Use!a!syringe!to!dispense!into!the!flask.!!Once!the!ether!is! added!to!the!flask,!immediately!connect!it!to!the!preassembled!apparatus!from!Step!2!to! minimize! exposure! of! the! reaction! mixture! to! atmospheric! moisture.! ! Also,! immediately! recap!(tightly!)!the!anhydrous!ether!reagent!can!to!minimize!exposure!to!the!atmosphere.!!! 4. After! clamping! the! reaction! apparatus! to! a! vertical! bar! in! the! hood,! place! a! magnetic! stirrer/hotplate! with! an! empty! porcelain! evaporating! dish! (to! be! used! as! a! warm! water! bath)! under! the! reaction! flask! and! lower! the! reaction! apparatus! so! that! the! round! bottom! flask! touches!the!bottom!of!the!evaporating!dish.! 5. Turn!on!the!water!to!the!reflux!condenser!and!start!the!magnetic!stirrer.! 6. Ask! your! TA! to! add! 1.0! mL! of! 3.0! M! ethereal! phenylmagnesium! bromide! dropwise! via! syringe! through!the!rubber!septum!to!your!stirred!reaction!mixture.!!An!exothermic!reaction!occurs!as! the! Grignard! reagent! is! added! to! the! methyl! benzoate! solution! and! the! ether! may! begin! to! reflux.! 7. Once! the! Grignard! reagent! has! been! added,! gently! reflux! (heat! to! gentle! boiling)! the! stirred! reaction!mixture!for!20!"25!minutes!by!adding!a!small!amount!of!the!previously!prepared!warm! water!to!the!evaporating!dish!water!bath!on!your!stirrer/hotplate.!!Keep!the!reaction!mixture! gently! refluxing! by! periodically! adding! more! warm! water! to! the! evaporating! dish.! ! If! you! heat! too!vigorously,!you!may!lose!varying!amounts!of!the!ether!solvent.! ! Page!1!of!2! ! CHM!2202! Organic!Chemistry!Lab!II! Spring!2010! ! Department!of!Chemistry! Villanova!University! ! 8. During!the!reflux!period,! magnesium!salts!will!form!and!the!reaction! mixture!will!form!a!solid! mass!which!may!not!be!able!to!be!stirred.! 9. After!the!20!minute!reflux!period,!remove!the!warm!water!bath!and!allow!the!reaction!mixture! to!cool!to!room!temperature.!!Remove!the!glassware!from!the!round!bottom!flask!and!add!the! following!materials!to!the!reaction!flask!in!the!following!order:! a. 2! mL! of! ether! (Do! not! use! the! anhydrous! ether,! but! the! regular! ether! which! is! also! provided!in!the!community!hood).! b. Several!pieces!of!ice.! c. Add!carefully!dropwise!and!with!stirring:!!~1.3!mL!of!10%!sulfuric!acid.! 10. After!the!sulfuric!acid!addition,!continue!to!magnetically!stir!the!contents!of!the!reaction!flask! until! the! solids! dissolve! to! give! two! clear! layers! (ether! and! aqueous).! ! You! may! want! to! use! a! spatula!to!break!up!any!large!chunks!of!solids!so!that!they!dissolve!faster.! 11. Pour!this!mixture!into!a!25!mL!separatory!funnel.! 12. Add!another!2!mL!of!ether!to!the!reaction!flask,!swirl!and!pour!the!ether!into!the!separatory!funnel.! 13. !Drain!off!the!lower!aqueous!layer!in!the!separatory!funnel!and!discard.! 14. Wash!the!remaining!ether!layer!by!adding!2!mL!of!water!to!the!separatory!funnel!and!shaking!gently.! Drain!off!the!lower!aqueous!layer!and!discard.! 15. Repeat!Step!14!using!2!mL!of!saturated!aqueous!sodium!chloride!(NaCl)!solution!instead!of!water.!Drain!off! the!lower!aqueous!NaCl!layer!and!discard.! 16. Pour! the! ether! layer! remaining! in! the! separatory! funnel! into! a! 25! mL! Erlenmeyer! flask.! Wash! the! separatory!funnel!with!1"2!mL!of!ether!and!add!the!ether!wash!to!the!Erlenmeyer.! 17. Dry!the!ether!by!adding!some!anhydrous!magnesium!sulfate!(MgSO4)!and!swirling!the!mixture.!!! 18. To!remove!the!MgSO4!drying!agent,!filter!the!ether!mixture!into!a!25!mL!Erlenmeyer!flask!through!a!fluted! filter!paper!placed!in!a!glass!short!stem!funnel.!Wash!the!drying!agent!remaining!in!the!flask!with!another!2! mL!portion!of!ether!and!filter!it!through!the!fluted!filter!paper!into!the!Erlenmeyer!containing!the!ether! solution!of!your!product.!!Using!a!disposable!pipette,!wash!the!wet!portion!of!the!fluted!filter!paper!with!2! mL!of!ether!to!dissolve!any!remaining!product!that!might!be!absorbed!in!the!filter!paper!and!drying!agent.! 19. Add!1!mL!of!high!boiling!petroleum!ether!and!one!boiling!stone!to!the!Erlenmeyer!flask!and!carefully! evaporate!most!of!the!ether!solvent!by!gently!heating!on!the!steam!bath.!! 20. When! most! of! the! ether! is! boiled! off,! the! triphenylmethanol! product! should! begin! to! crystallize! out! of! solution! as! white! crystals.! When! this! happens,! remove! the! flask! from! the! steam!bath!and!complete!the!crystallization!process!by!cooling!the!flask!in!ice.!You!may!want!to! add!another!1"2!mL!of!petroleum!ether!if!there!is!not!much!solvent!remaining!in!the!flask.! 21. Filter!off!the!crystalline!product!using!vacuum!filtration!and!your!small!porcelain!Hirsch!funnel.!If! necessary,!use!some!cold!high!boiling!petroleum!ether!to!assist!in!washing!out!the!remaining! crystals!from!the!recrystallization!flask.!Dry!the!crystals!on!the!funnel!by!sucking!air!through!the! crystals!for!several!minutes!until!they!appear!to!be!dry!and!free!flowing.! 22. Weigh!the!product!and!determine!its!melting!point!range!(compare!with!the!literature!value)!and!%! yield.! 23. If!the!melting!point!range!of!your!material!differs!more!than!10!–!15!degrees!from!the!expected! melting!point!of!triphenylmethanol,!take!an!IR!spectrum!(ask!a!TA!for!assistance)!of!your!product! and!compare!it!to!the!IR!of!the!authentic!product!(provided!as!a!handout).! 24. Also!interpret!the!IR,!13C!and!1H!NMR!spectra!(provided!as!handouts)!of!methyl!benzoate!and!the! triphenylmethanol!product.!Assign!key!absorption!bands/peaks!in!the!IR!and!NMR!spectra.! 25. Be!sure!to!include!the!spectra!with!pertinent!peaks!labeled!with!your!observations!when!handing! in!your!assignments.! 26. Disassemble!your!drying!tube!by!pouring!the!drying!agent!into!the!bottle!specifically!provided!for! Used!Drying!Agent!and!returning!the!one!hole!rubber!stopper!to!the!supplies!area!(blue!bins)!at! the!back!of!the!labs.! TMBare!(1/20/2010)! Page!2!of!2! !