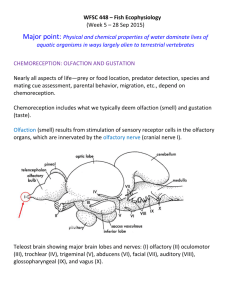
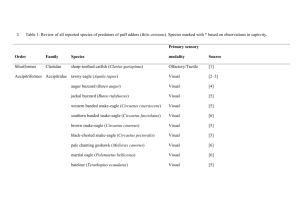
Anatomy and Physiology of the Elasmobranch Olfactory System
advertisement

ANATOMY AND PHYSIOLOGY OF THE ELASMOBRACH OLFACTORY SYSTEM by Tricia L. Meredith A Dissertation Submitted to the Faculty of The Charles E. Schmidt College of Science in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Florida Atlantic University Boca Raton, FL December 2011 ACKNOWLEGMENTS This work would not have been possible without financial support from several private donors and scientific organizations including: Gordon Gilbert Scholarship, Captain Al Nathan Memorial Scholarship, Edward Shoaf Scholarship, Marsh Scholarship in Marine Biology, Donald R. Nelson Behavior Research Award, Helen O‟Leary Scholarship, Dr. Vincent R. Saurino Fellowship, American Elasmobranch Society Student Research Award, Lerner-Gray Grant for Marine Research, and Sigma Xi Grants-in-Aid of Research. Additionally, internal support from Florida Atlantic University (FAU) included the FAU Graduate Fellowship for Academic Excellence, FAU Memorial Scholarship, FAU Student Government Scholarship for Undergraduates and Graduates, FAU Alumni Association Scholarship, and a teaching assistantship each semester. While the many funding sources provided the means, several people provided the guidance along this amazing journey. I must first thank my advisor, Dr. Stephen Kajiura. He introduced me to the world of olfaction research during a research trip to Hawaii where I learned everything from shark fishing to EOG electrode construction. From there he mentored me through my Ph.D., providing invaluable advice, edits, and most importantly the basic tools necessary to figure things out for myself in almost any situation. The other members of my dissertation committee, Dr. Kathleen Guthrie, Dr. John Baldwin, Dr. Sarah Milton, Dr. John Caprio (LSU), were also very supportive throughout this process. I cannot express enough gratitude toward John Caprio in particular. He took me under his wing during a time when I was in need of guidance and another perspective, helped me carve a definitive path to obtaining my doctoral degree, and has been there for me every single step of the way. Dr. Tricas allowed me to invade his lab on Coconut Island in Oahu, HI twice to learn electro-physiological techniques and participate in interesting projects. The Gumbo Limbo Environmental Complex in Boca Raton, Fl provided me with use of iii their facility, and the staff, including Kirt Rusenko and Neal Tempel, was always available to help troubleshoot any issues that arose. Bob Hueter, Jack Morris, Carl Luer, and Jayne Gardiner, at Mote Marine Laboratory in Sarasota, Fl, also supplied me with animals, tank space, and lab space. Doug Adams, Joy Young, and colleagues at the Florida Fish and Wildlife Conservation Commission were instrumental in helping me obtain many of my experimental specimens. Dr. Anne Hansen not only welcomed me into her lab at the University of Colorado Denver (and her home) twice, but also was an invaluable mentor. I thank Dr. Rod Murphey, Dr. Evelyn Frazier, Dr. Ken Dawson-Scully, Geri Mayer, and Jenny Govender from the FAU Biological Sciences Department for all of the assistance and opportunities they provided. I also could not have survived graduate school without my labmates and fellow graduate students. Thanks to Dr. M. Porter, M. Smith, L. Dirk, A. Cornett, L. Macesic, M. McComb, D. McGowan, C. Bedore, L. Harris, K. Smith, S. McCutcheon, and the many volunteers that donated their time to my research. Through all of the coffee breaks, edits, happy hours, lab meetings, field trips, and classes, you helped keep me sane. Finally, I thank my family for all of their love and support. Mom, Dad, Grandma Greathouse, Grandma and Grandpa Meredith, thank you for teaching me at an early age that I can do whatever I want in my life as long as I work hard to achieve it. Even if it‟s playing in the ocean for a living! I also thank my sisters who inspire me to be a worthy role model and my amazing fiancé, Dave, who has been nothing but supportive from beginning to end. iv ABSTRACT Author: Tricia L. Meredith Title: Anatomy and Physiology of the Elasmobranch Olfactory System Institution: Florida Atlantic University Dissertation Advisor: Dr. Stephen M. Kajiura Degree: Doctor of Philosophy Year: 2011 The olfactory system is the most highly developed system for molecular sensing in vertebrates. Despite their reputation for being particularly olfactory driven, little is known about how this sense functions in elasmobranch fishes. The goal of this dissertation was to examine the morphology and physiology of elasmobranchs to compare their olfactory system with teleost fishes and more derived vertebrates. To test the hypotheses that elasmobranchs possess greater olfactory sensitivities than teleosts and that lamellar surface area is correlated to sensitivity, I compared the surface area of the olfactory lamellae and the olfactory sensitivities of five phylogenetically diverse elasmobranch species. The olfactory thresholds reported here (10 –6 to 10 –9 M) were comparable to those previously reported for teleosts and did not correlate with lamellar surface area. Since aquatic species are subject to similar environmental amino acid levels, they appear to have converged upon similar amino acid sensitivities. To test the hypothesis that elasmobranchs are able to detect bile salt odorants despite lacking ciliated olfactory receptor neurons (ORNs), the type of ORN that mediates bile salt detection in the teleosts, I quantified the olfactory specificity and sensitivity of two elasmobranch species to four, teleost-produced C24 bile salts. Both species responded to all four bile salts, but demonstrated v smaller relative responses and less sensitivity compared to teleosts and agnathans. This may indicate that elasmobranchs don‟t rely on bile salts to detect teleost prey. Also, the olfactory system of elasmobranchs contains molecular olfactory receptors for bile salts independent of those that detect amino acids, similar to teleosts. In some elasmobranch species, each olfactory bulb (OB) is physically partitioned into two hemi-bulbs; however, the functional significance of this morphology is not fully understood. The organization of the OBs in three species with varying OB morphologies was examined to test the hypothesis that the elasmobranch OB is somatotopically arranged. Glomeruli in the OB received projections from ORNs in 3-4 olfactory lamellae situated immediately anterior. These results indicate a somatotopic arrangement of the elasmobranch OB, which may be unique among vertebrate olfactory systems and potentially led to the hemi-OB morphology. vi ANATOMY AND PHYSIOLOGY OF THE ELASMOBRANCH OLFACTORY SYSTEM LIST OF TABLES ............................................................................................................................. x LIST OF FIGURES .......................................................................................................................... xi CHAPTER 1 ..................................................................................................................................... 1 CHEMORECEPTION ................................................................................................................... 1 CONSERVED FEATURES IN VERTEBRATE OLFACTION....................................................... 2 OLFACTION IN FISHES .............................................................................................................. 3 Morphology of the teleost olfactory system .............................................................................. 4 Morphology of the elasmobranch olfactory system ................................................................. 7 Physiology of the teleost olfactory system ............................................................................. 11 Physiology of the elasmobranch olfactory system ................................................................. 14 REFERENCES ........................................................................................................................... 17 CHAPTER 2 ................................................................................................................................... 29 ABSTRACT ................................................................................................................................ 29 INTRODUCTION........................................................................................................................ 29 MATERIALS AND METHODS ................................................................................................... 31 Animal Collection ................................................................................................................... 31 Morphology............................................................................................................................. 31 Electrophysiology: Experimental Protocol .............................................................................. 33 Analysis .................................................................................................................................. 35 RESULTS ................................................................................................................................... 36 Morphology............................................................................................................................. 36 vii Electrophysiology: Relative effectiveness of amino acids ..................................................... 36 Electrophysiology: Concentration-response relationships and olfactory threshold ............... 37 DISCUSSION ............................................................................................................................. 37 REFERENCES ........................................................................................................................... 43 CHAPTER 3 ................................................................................................................................... 56 ABSTRACT ................................................................................................................................ 56 INTRODUCTION........................................................................................................................ 56 MATERIALS AND METHODS ................................................................................................... 59 Animal Collection ................................................................................................................... 59 Relative effectiveness ............................................................................................................ 61 Concentration-response relationships ................................................................................... 61 Cross-adaptation .................................................................................................................... 61 RESULTS ................................................................................................................................... 63 Relative effectiveness ............................................................................................................ 63 Cross-adaptation .................................................................................................................... 63 DISCUSSION ............................................................................................................................. 64 REFERENCES ........................................................................................................................... 69 CHAPTER 4 ................................................................................................................................... 81 ABSTRACT ................................................................................................................................ 81 INTRODUCTION........................................................................................................................ 81 METHODS ................................................................................................................................. 85 Sample collection ................................................................................................................... 85 Histological staining ............................................................................................................... 85 Retrograde tracing ................................................................................................................. 86 RESULTS ................................................................................................................................... 86 Morphology............................................................................................................................. 86 Organization of ORN projections ........................................................................................... 87 viii DISCUSSION ............................................................................................................................. 88 Morphology............................................................................................................................. 88 Organization of ORN projections ........................................................................................... 91 REFERENCES ........................................................................................................................... 94 CHAPTER 5 ................................................................................................................................. 105 ELASMOBRANCH OLFACTION RESEARCH ........................................................................ 105 RESULTS OF THE PRESENT STUDY ................................................................................... 107 Chapter 2: Olfactory morphology and physiology ................................................................ 107 Chapter 3: Sensitivity and specificity to bile salts ................................................................ 108 Chapter 4: Somatotopy of the hemi-olfactory bulb .............................................................. 110 FUTURE RESEARCH .............................................................................................................. 111 Understanding elasmobranchs ............................................................................................ 111 Understanding olfaction ....................................................................................................... 112 REFERENCES ......................................................................................................................... 114 ix LIST OF TABLES Table 2.1 The number and appearance of the olfactory lamellae for the species used in this study. ................................................................................................................................. 48 x Table 2.2 Mean olfactory thresholds (10 M) of five elasmobranch species to highly stimulatory amino acids after compensation for stimulus dilution. ...................................................... 49 Table 2.3 Ranked mean EOG responses of five elasmobranch species to 20 amino acids. ........ 50 x Table 3.1 Mean olfactory thresholds (10 M ± s.e.m.) to four bile salts after compensation for stimulus dilution. ................................................................................................................ 75 x LIST OF FIGURES Figure 2.1 Experimental apparatus used to record the electro-olfactogram (EOG). Animals were secured onto a platform in an experimental seawater (SW) tank and ventilated with SW. The tank was supplied with mechanically and chemically filtered SW through a PVC manifold. One manifold arm delivered a constant flow of SW through a flow meter; paired computer-controlled, three-way solenoid valves; and an odor delivery pipette (ODP, inset), which was inserted into the incurrent naris. Amino acid stimuli were delivered through the ODP to the olfactory organ. Paired, non-polarizable, Ag-AgCl electrodes recorded the EOG. The glass tip of the active electrode (Act E, inset) was positioned in the SW immediately above the olfactory epithelium while the glass tip of the reference electrode (Ref E, inset) contacted the skin adjacent to the naris. The output was differentially amplified (1000-10000x), filtered (high pass 0.1 Hz, low pass 0.1 kHz, 50/60 Hz), digitized (1 kHz) and recorded. ................................................................................. 51 Figure 2.2 Total lamellar surface area for the five elasmobranch species tested scaled positively as a power function with body size (disc width or total length). Regression lines are 1.8988 2.4689 indicated for R. eglanteria: y = 0.0402x ; U. jamaicensis: y = 0.0297x ; D. sabina: 1.5725 1.4371 1.3342 y = 0.242x ; N. brevirostris: y = 0.0947x ; S. tiburo: y = 0.3052x . Outliers were excluded from the regressions for R. eglanteria, D. sabina, and N. brevirostris. Raja eglanteria had a significantly smaller mean total lamellar surface area than U. jamiacensis and D. sabina. Negaprion brevirostris had a significantly smaller mean total lamellar surface area than S. tiburo. ................................................................................. 52 Figure 2.3 Mean EOG responses (+ s.d.) for five elasmobranch species (n≥6) to 20 amino acids and the SW control. Response magnitudes are expressed as a percent of the response -3 to the standard (10 M alanine). Colors represent the results of pair-wise comparisons using Bonferroni t-tests. For each species, amino acids that share a bar color do not differ significantly from each other. ala alanine, arg arginine, asn asparagine, asp aspartic acid, cys cysteine, gln glutamine, glu glutamic acid, gly glycine, his histidine, ile isoleucine, leu leucine, lys lysine, met methionine, phe phenylalanine, pro proline, ser serine, thr threonine, trp tryptophan, tyr tyrosine, val valine, sw seawater. Line drawings of batoids are modified from McEachran and de Carvalho (2002), and line drawings of sharks are modified from Compagno (2002). ................................................................... 53 Figure 2.4 Concentration-response relationships of five or six highly stimulatory amino acids for each species. Response magnitudes represent the mean response for each amino acid -3 at each concentration and are expressed as a percent of the standard (10 M alanine). Refer to Fig. 3 for statistical differences............................................................................ 54 Figure 2.5 Representative EOG concentration-response curve of a S. tiburo individual expressed -3 as a percent of the standard (10 M alanine). The magnitude of the log EOG response is linearly related to the log amino acid stimulus concentration. The horizontal dashed black line indicates the averaged response to the seawater control. The olfactory threshold is calculated as the point where the regression line for the best fit line of the response xi intersects the averaged response to the seawater (SW) control. The inset shows representative EOG responses to the SW control and to increasing log concentrations of L-alanine. Based on absorbance calculations of diluted dye, all stimuli were diluted to 6% of their injected concentration at the entrance to the incurrent naris. Plotted are the estimated diluted stimulus concentrations at arrival to the olfactory organ. ..................... 55 Figure 3.1 Experimental apparatus used to record the electro-olfactogram (EOG) during crossadaptation experiments. Animals were secured to a platform in an experimental tank filled with seawater (SW). One arm of a PVC manifold delivered ventilatory SW flow over the animal„s gills, and a second manifold arm supplied the tank with SW. A small pump delivered a constant flow of either SW or adapting amino acid (AA) solution through a flow meter and to an odor delivery pipette (ODP). Bile salt stimuli were injected into the tubing immediately above the ODP and transported to the olfactory organ. Paired, nonpolarizable, Ag-AgCl electrodes recorded the EOG; the glass tip of the active electrode (Act E, inset) was positioned in the SW immediately above the olfactory epithelium, whereas the glass tip of the reference electrode (Ref E, inset) contacted the skin. The output was differentially amplified (1000x), filtered (high pass 0.1 Hz, low pass 0.1 kHz, 50/60 Hz), digitized (1 kHz) and recorded. ....................................................................... 76 Figure 3.2 Protocol for the cross-adaptation experiments. EOG responses were recorded to four bile salts, representing nonconjugated (NC), glycine-conjugated (GC), and taurineconjugated (TC) groups (A.), delivered individually over a background flow of seawater (SW) (B.). This pulse of bile salt odorant elicits a phasic EOG response (C.). The background flow was then randomly switched to one of four adapting amino acids, each representing a side-chain structural group. The adapting amino acid was presented continuously over the olfactory epithelium for ten minutes resulting in a tonic EOG response during which phasic responses to the bile salts were recorded. This phasic response is only seen if the test stimulus interacts with independent receptors from those for the adapting stimulus. The background flow was returned to SW for thirty minutes to allow the olfactory receptors to unadapt; and the EOG responses to the bile salts were again recorded to confirm that the animal was still responding at the pre-adaptation level. This procedure was repeated for each of the three remaining adapting regimes. The molecular structures for each bile salt, modified from Zhang and Hara (2009), are provided to the left. TLCS taurolithocholic acid 3-sulfate, TCDC taurochenodeoxycholic acid, GCDC glycochenodeoxycholic acid, CA cholic acid. ............................................... 77 Figure 3.3 Mean EOG responses (+ s.d.) for D. sabina (n≥5, black bars) and S. tiburo (n=4, white -4 bars) to four bile salts at an injection concentration of 10 M and two controls, SW and 0.1% MeOH. Response magnitudes are expressed as a percent of the response to the -3 standard (10 M alanine). Letters within each bar represent the results of pair-wise comparisons using Holm-Sidak tests. The mean EOG response magnitudes to the four bile salts varied significantly for both species and ranged from 7-18% of the response to the standard. For each species, bile salts that share a letter do not differ significantly from each other. TLCS taurolithocholic acid 3-sulfate, TCDC taurochenodeoxycholic acid, GCDC glycochenodeoxycholic acid, CA cholic acid. ............................................... 78 Figure 3.4 Concentration-response relationships of four bile salts for D. sabina and S. tiburo. Response magnitudes represent the mean response for each bile salt at each -3 concentration and are expressed as a percent of the standard (10 M alanine). The EOG response increased predictably with increasing bile salt concentration. Line drawing of D. sabina is modified from McEachran and de Carvalho (2002), and line drawing of S. tiburo is modified from Compagno (2002). TLCS taurolithocholic acid 3- xii sulfate, TCDC taurochenodeoxycholic acid, GCDC glycochenodeoxycholic acid, CA cholic acid. ........................................................................................................................ 79 Figure 3.5 Mean percent unadapted responses (PUR) (+ S.E.) for D. sabina and S. tiburo to four bile salts presented individually to the olfactory epithelium during each of four L-amino acid adapting regimes. Percent unadapted responses did not significantly differ among bile salts or among amino acid adapting regimes for either species. The dotted line indicates 100% PUR. Line drawing of D. sabina is modified from McEachran and de Carvalho (2002), and line drawing of S. tiburo is modified from Compagno (2002). TLCS taurolithocholic acid 3-sulfate, TCDC taurochenodeoxycholic acid, GCDC glycochenodeoxycholic acid, CA cholic acid. ................................................................... 80 Figure 4.1 The three species used in this study represent two elasmobranch orders; Rajiformes (stingrays) and Carcharhiniformes (shark) (A). Photographs of the brains of the Atlantic stingray (D. sabina) and lemon shark (N. brevirostris) illustrate their differing OB morphologies. The OBs of D. sabina and D. say, which both occur as a cohesive unit, whereas the olfactory bulb (OB) of N. brevirostris occurs as two physically separate hemiolfactory bulbs (B). A schematic diagram of the olfactory pathway in elasmobranchs (C) shows the olfactory organ that is comprised of several lamellae overlain with the secondarily folded olfactory epithelium (OE). Odorants that enter the olfactory capsule bind to molecular olfactory receptors (ORs) on the microvilli and cilia of the microvillous (blue) and crypt (green) olfactory receptor neurons (ORNs) in the OE. The ORN axons project through the lamina propria of each lamella, through the short olfactory nerve (not visible externally), and to the OB where they synapse with mitral cells at glomeruli. Line drawings of the Dasyatids are modified from McEachran and de Carvalho (2002), and the line drawing of the lemon shark is modified from Compagno (2002). The schematic in panel C is modified from Hamdani and Døving (2007) and Firestein (2001). ................ 100 Figure 4.2 Morphology of the OB and OE of D. say visualized using KLO (nuclear red-light greenorange) staining. An inset in the top left corner illustrates the area of the olfactory bulb and organ being examined (gray shaded area). The axons of the ORNs situated in the folds of OE project through the lamina propria of each lamella and to the OB where they synapse with mitral cells at glomeruli. The laminar organization of the OB is apparent. A superficial connective tissue layer occurred at the posterior aspect of the OB. Deep to that layer, glomeruli (*) were distributed in a diffuse layer and arranged around the axons (dotted circle). ................................................................................................................. 101 Figure 4.3 Retrograde labeling from the OB to the OE in both stingray species. An inset in the top left corner of each panel illustrates the dye type (green = Far Red and red = DiI), general dye placement location, and the area of the olfactory bulb and organ being examined (gray shaded area). Panels A and B show labeling of the glomeruli (*) and axons in the OB near the labeling site. DiI in the OB spread into the adjacent nerve fascicles and the axon bundles in the lamina propria of the three to five lamellae immediately anterior to the labeling site in the OB (Panel C). Labeling extended from those axon bundles into axons innervating the ORNs in the secondary folds of the OE (Panels D-F). Panel G shows a single microvillus ORN labeled with DiI. No labeling was seen in distant lamellae. ................................................................................................. 102 Figure 4.4 Retrograde labeling from the OB to the OE in the lemon shark, N. brevirostris. An inset in the top left corner of each panel illustrates the dye type (green = Far Red and red = DiI), general dye placement location, and the area of the olfactory bulb and organ being examined (gray shaded area). Panel A shows distinct labeling of the medial and lateral hemi-OBs. Panel B shows a DiI labeled medial hemi-OB, including axon bundles from xiii the adjacent olfactory lamellae leading to several glomeruli (*). Panel C is a composite image of multiple olfactory lamellae labeled on the medial side with DiI and the lateral side with Far Red. No double labeling of lamellae was seen. Panels D and E show the olfactory epithelium and individual ORNs labeled with DiI. The sample in panel D was labeled with both dyes in the lateral hemi-OB; however, we saw no double labeling of the OE. .................................................................................................................................. 103 Figure 4.5 Retrograde labeling from the OB to the OE in lemon shark (N. brevirostris) samples that were labeled using both dyes in a single hemi-OB. An inset in the top left corner of each panel illustrates the dye type (green = Far Red and red = DiI), general dye placement location, and the area of the olfactory bulb and organ being examined (gray shaded area). Although the lateral hemi-OB was labeled with both dyes in the sample in Panel A, there was distinct labeling of axon bundles by each dye. In contrast, only when the two dyes were placed so closely that the same axons were affected we observed double labeling of axons in the OB, indicated by the yellow hue (Panel B). In samples where both dyes were placed in the same hemi-OB, we did not see double labeling of any lamellae or ORNs. .................................................................................................... 104 xiv CHAPTER 1 INTRODUCTION CHEMORECEPTION Chemoreception and mechanoreception are hypothesized to be the two earliest receptor systems from which all other sensory receptors were derived (Hodos and Butler, 1997). Chemical sensitivity, which occurs in virtually all organisms, from single-celled bacteria to humans, enables organisms to detect and respond to chemical stimuli in their environment. Even within our own bodies, each living cell must be sensitive to important molecules in its local environment. The chemoreceptive systems found in vertebrates include olfaction, gustation, and the common chemical sense. The olfactory system, which uniquely uses cranial nerve I, is the most highly developed system for molecular sensing in vertebrates, and is astounding in its ability to sense and distinguish among thousands of low molecular mass, mainly organic compounds, called odors (Ache and Young, 2005; Firestein, 2001). Many similarities exist in the organization of the olfactory pathways in a phylogenetically diverse array of animals (Ache and Young, 2005; Firestein, 2001). In both terrestrial and aquatic vertebrates, odorants make contact with olfactory receptors via a fluid medium covering the olfactory receptor cells, reflecting the evolutionary origin of olfaction in an aquatic environment (Strausfeld and Hildebrand, 1999). Whether the olfactory system similarities are the result of homology or evolutionary convergence seems ambiguous (Strausfeld and Hildebrand, 1999). However, phylogenetic analyses indicate that several conserved features are not homologous, and thus may represent convergent adaptations, responses to similar constraints, and adaptations for similar tasks (Eisthen, 2002). 1 CONSERVED FEATURES IN VERTEBRATE OLFACTION Odor detection in vertebrates begins when odorants enter through either a nostril or naris into the olfactory cavity, which houses a sheet of olfactory epithelium (OE). Odorant molecules bind to G-protein coupled olfactory receptors (ORs) on the cilia or microvilli of olfactory receptor neurons (ORNs) in the mucus-bathed OE (Eisthen, 2002). These ORNs are primary sensory neurons that project directly to the brain and regenerate throughout an animal‟s life. Odorant binding activates G-proteins inside the ORN, which in turn activates second messenger pathways, primarily the adenylate cyclase (AC)/cyclic-AMP (cAMP) pathway or the phospholipase + 2+ C (PLC)/inositol triphosphate (IP3) pathway to open ion channels. Positive ions (Na , Ca ) influx and accumulate inside the cell and may secondarily open calcium-activated chloride channels leading to chloride ion efflux, resulting in ORN depolarization. When the depolarization of an ORN exceeds threshold, action potentials are generated and conducted along the ORN axon, which converges with the other ORN axons to form the olfactory nerve (cranial nerve I). These axons then project to the olfactory bulb (OB) of the forebrain where they synapse with mitral cells in a spherical bundle of neuropil, termed glomeruli (Ache and Young, 2005; Firestein, 2001). Receptor cell morphology is strongly correlated with odorant receptor gene expression and somewhat less strictly to G-protein expression. In vertebrates, ciliated ORNs express receptor proteins from the OR gene family utilizing the Gα olf transduction cascade and microvillous ORNs express V2R-family receptors coupled to either Gαo, Gαq, or Gαi-3 (Eisthen, 2004). Most fishes, including lampreys, elasmobranchs, and actinopterygians, possess a third morphological ORN type. These are crypt ORNs that express Gαo, Gαq, or sometimes coexpress both within a given cell; the receptor gene expression in crypt ORNs is still unknown (Ferrando et al., 2006; Hansen et al., 2004; Laframboise et al., 2007; Zeiske et al., 2003). Individual ORNs in the OE express only one out of approximately 1000 (in mammals, about 100 in fish) olfactory receptor genes (Buck and Axel, 1991; Firestein, 2001). ORNs that express the same specific receptor gene, which determines their specificity to particular odors, are distributed throughout the sensory OE; however their axons converge to synapse at one or a few localized 2 glomeruli in the OB. The OB is divided into separate functional regions that each process information about different odorant classes thereby creating an odotopic, or chemotopic, OB map. As a result, odorant identity and concentration are encoded by a spatial pattern of glomerular activation (Bozza and Kauer, 1998; Caprio and Derby, 2008; Friedrich and Korsching, 1997; Hildebrand and Shepherd, 1997; Johnson and Leon, 2007; Strausfeld and Hildebrand, 1999; Xu et al., 2000). Due to its role in encoding olfactory information, chemotopy is found in the OB of many vertebrates, including teleosts, amphibians, reptiles, and mammals (Hildebrand 1997). Animal models, such as those mentioned above, are useful not only for investigating olfaction as it applies to more derived vertebrates, but also for understanding the evolution of this ancient sensory modality. The research presented in this dissertation focuses on olfaction in elasmobranch fishes (sharks, rays, and skates). Elasmobranch fishes make excellent models for research on vertebrate olfaction for several reasons. They have persisted in the vertebrate clade for over 450 million years yielding over 800 extant species (Janvier, 1996). These species occupy a wide variety of ecological niches, each imposing a unique suite of selective pressures that have potentially shaped the evolution of this sensory system (Hueter et al., 2004). As a result, elasmobranchs possess great interspecific diversity in their olfactory morphology, with largely undetermined consequences on their olfactory physiology and olfactory-mediated behavior. Though much basic research is lacking, sharks are renowned for their sensory capabilities. Many assumptions about their olfactory capabilities in particular are drawn either from anecdotal evidence or from research on their more extensively researched relatives, the more basal agnathans and the more derived teleost fishes. OLFACTION IN FISHES Agnathans are jawless fishes that are basal to elasmobranchs in the vertebrate clade. Modern representatives, hagfish and lampreys, rely heavily on olfaction for many critical life history functions. In addition, the sea lamprey, Petromyzon marinus, is a fish parasite that has invaded the Great Lakes of North America causing the collapse of several fisheries (Smith and Tibbles, 1980). As a results, pheromones as natural chemical attractants were explored as a 3 method to control sea lamprey populations, causing a proliferation of agnathan olfactory studies (Sorensen et al., 2005; Stacey and Sorensen, 2005); however, these studies are generally limited to investigations of pheromones and their practical application in management strategies and generally do not include other biologically relevant odorant classes. Teleost fishes, which are more derived compared to elasmobranchs, are a group of bony fishes comprised of most of the extant fish species. With well over 23,000 current species, teleosts are the most numerous and diverse of the major vertebrate groups (Moyle and Cech, 2004). Olfaction is a vital chemosensory modality for teleosts as it mediates nearly every major life history function, including feeding, reproduction, migration, and predator detection (Carr et al., 1989; Sorensen and Caprio, 1997; Tester, 1963; Zielinski and Hara, 2006). Consequently, the teleost olfactory system exhibits considerable morphological diversity and responds with remarkable specificity and sensitivity to a wide variety of chemicals in the aquatic environment. In addition, due to the relative ease of collection and maintenance in captivity (especially compared to elasmobranchs), olfactory research in teleosts has flourished. Although a relative void exists for research in elasmobranch olfaction, new findings can be readily placed into an evolutionary context, thus adding to the benefits of studying elasmobranchs. Morphology of the teleost olfactory system In contrast to agnathans, which possess a single median nostril leading to a single olfactory cavity, teleosts fishes possess paired olfactory capsules. Each capsule is connected to two nares, located on the dorsal side of the head rostral to the eyes, which facilitates a unidirectional flow of water. Odorant-laden water flows into the olfactory capsule through the anterior naris and exits via the posterior naris (Zielinski and Hara, 2001). Unlike terrestrial vertebrates, there is no connection between the olfactory capsule and the respiratory system, so in a group of fishes called isosmates, irrigation of the olfactory capsule is achieved through use of external flows (e.g. swimming or rheotaxis) and the ciliary action of non-sensory cells (Cox, 2008; Døving et al., 1977). Cyclosmates, which are more sedentary, demersal species, possess one or two accessory nasal sacs, which they expand and compress to aid in olfactory capsule irrigation, 4 a process which has been compared to “sniffing” behavior in mammals (Belanger et al., 2003; Cox, 2008; Døving et al., 1977; Nevitt, 1991). The olfactory capsule houses the peripheral olfactory organ, which is comprised of platelike lamellae overlain with the OE. Teleost fishes exhibit a wide diversity in the number and arrangement of the olfactory lamellae and in the surface area of the OE (Hansen and Zielinski, 2005; Kleerekoper, 1969; Yamamoto, 1982). For example, killifish (Cyprinodontiformes) species possess a flat, unilamellar, or multilamellar OE with corresponding increases in epithelial surface area (Hansen and Zielinski, 2005). In the order Perciformes, the number of olfactory lamellae ranges from zero to 64 (Yamamoto, 1982). These variations in teleost olfactory organ morphology were hypothesized to correlate with either the ecology or phylogeny (Yamamoto, 1982; Zielinski and Hara, 2001); however, in their survey of olfactory morphology of over 70 teleost species, Hansen and Zielinski (2005) found that the number of lamellae correlated with neither phylogeny nor ecology, and they could not provide an alternative explanation for the wide variation in lamellar configuration. In addition, the presence of more lamellae or greater epithelial surface area was thought to correlate with olfactory sensitivity by increasing the surface area for odorant binding. Yet, teleost species with a flat OE still demonstrate comparable olfactory responses to those with multilamellar olfactory organs and more epithelial surface area (Zielinski and Hara, 2006). The sensory epithelium in the teleost olfactory organ is comprised of pseudostratified columnar epithelium populated with the same three cells types found in the OE of all vertebrates: supporting cells, basal cells, and ORNs (Byrd and Brunjes, 1995; Hansen and Zeiske, 1998). Mucus secreting goblet cells may also occur in the sensory OE depending on the species (Hansen and Zielinski, 2005). Teleosts possess three morphological types of ORNs: ciliated, microvillous, and crypt cells. Ciliated and microvillous ORNs are common in vertebrates; however, crypt ORNs have only been documented in lampreys, elasmobranchs, and actinopterygian (but not sarcoptergygian) fishes (Ferrando et al., 2006; Hansen and Finger, 2000; Laframboise et al., 2007). Both ciliated and microvillous ORNs are spindle-shaped cells whose 5 cell bodies occupy different layers of the OE. The somata of ciliated ORNs are located in the basal portion of the OE, and microvillous ORN somata occupy the upper two-thirds of the OE (Hansen and Zielinski, 2005). The dendrites of ORNs terminate apically in an olfactory knob from which either cilia or microvilli extend into the lumen of the olfactory cavity. Crypt ORNs are oval or ovoid- shaped cells located in the upper third of the OE in an invagination, or crypt. Cilia project from the cell into this crypt, which is surrounded by microvilli (Hansen and Finger, 2000). The three ORN types each express a single molecular OR type coupled to a specific Gprotein α subunit and are sensitive to a distinct suite of odorants (Hansen et al., 2003). As seen in other vertebrates, ciliated ORNs express the OR-type receptor utilizing the Gαolf transduction cascade, and microvillous ORNs expressing the V2R-type receptor coupled to either Gαo, Gαi, or Gαq (Eisthen, 2004). The receptor gene expressed in crypt ORNs is unknown, but has been shown to express Gαo or Gαq (Ferrando et al., 2006; Hansen et al., 2004; Hansen and Finger, 2000; Laframboise et al., 2007; Zeiske et al., 2003). The different ORN morphotypes are thought to be randomly distributed throughout the sensory epithelium, but in some species a distinct pattern is detectable (Hansen and Zielinski, 2005). For example, in goldfish, microvillous ORNs are clustered in the dorso-medial regions of the lamellae rather than being distributed throughout the lamellae (Hansen et al., 2004). Axons of ORNs that express the same specific receptor gene, which dictates their specificity to particular odors, converge to synapse at one or a few localized glomeruli in the OB. This creates a chemotopic OB organization, which has been documented in most other vertebrate groups (Baier et al., 1994; Døving et al., 2011; Hansen et al., 2003; Morita and Finger, 1998; Sato et al., 2005). The teleost OB exhibits interspecific differences in terms of placement relative to other structures, which may manifest as one of two forms. In some species, such as the salmonids, the olfactory organ is connected by a long olfactory nerve to a sessile OB, which is located adjacent to the telencephalon, joined via a short olfactory tract. In contrast, in species such as the cyprinids, the olfactory organ is connected by a short olfactory nerve to the adjacent pedunculated OB, which is connected to the telencephalon by a long olfactory tract (Zielinski and 6 Hara, 2006). OB placement variations aside, its cytoarchitecture is similar to that of other vertebrates. The OB is comprised of four concentric layers that serve to integrate incoming sensory input and deliver that information to higher telencephalic areas for memory formation or motor responses (Byrd and Brunjes, 1995; Hamdani and Døving, 2007; Laberge and Hara, 2001; Oka et al., 1982). The olfactory nerve layer, which is most superficial, consists of the axons of ORNs. In the next layer, the glomerular layer, ORN axon terminals synapse with mitral cell dendrites, forming spherical bundles of neuropil, termed glomeruli, that in species such as the zebrafish and goldfish, are arranged in a stereotyped pattern (Baier and Korsching, 1994). Each mitral cell may synapse at a single or multiple glomeruli (Dryer and Graziadei, 1994a; Satou, 1992; Zielinski and Hara, 2006). The mitral cell somata are located in the next deep layer, called the mitral cell layer, and their axons project through the olfactory tract to the telencephalon. Glomeruli and mitral cell somata comingle in the OBs of certain species, sometimes making these two layers indistinguishable. The granule cell layer, which is the deepest layer, contains granule cells, which lack axons but make dendritic synapses with mitral cells. The granule cells are inhibitory interneurons thought to modulate mitral cell activity. The olfactory tract, comprised of bundles of mitral cell axons, is divided into distinct medial and lateral components that project to separate telencephalic regions that are thought to mediate different behaviors (Døving and Selset, 1980; Hamdani et al., 2001; Hamdani et al., 2000; Weltzien et al., 2003). The medial tract transmits pheromonal information whereas the lateral tract transmits food-related information. Morphology of the elasmobranch olfactory system Just as with other vertebrates, olfaction plays an integral role for elasmobranchs in food localization (Parker, 1913; Sheldon, 1909; Sheldon, 1911) and also likely in mating (Johnson and Nelson, 1978; Kajiura et al., 2000), predator detection (Rasmussen and Schmidt, 1992), and homing and navigation (Edrén and Gruber, 2005). The olfactory morphology of elasmobranchs is fundamentally similar to that described above for teleosts, but some important differences between these two fish groups will be highlighted. 7 Odor detection for elasmobranchs begins when dissolved chemicals pass into paired incurrent nares located on either the terminal or subterminal surface of the head, rather than dorsally as seen in teleosts (Tester, 1963). The nasal opening is incompletely divided into a lateral incurrent opening and a medial excurrent opening to facilitate a unidirectional flow of water through the olfactory capsule (Tester, 1963). Elasmobranch species exhibit a wide diversity in their external olfactory morphology, which may correlate with species‟ phylogeny or ecology (Bell, 1993; Kleerekoper, 1978; Meng and Yin, 1981; Tester, 1963). For example, all eight species in the hammerhead family, Sphyrnidae, possess a unique, laterally expanded head morphology, termed the cephalofoil, in which the nares are positioned at the distal tips of the head (Lim et al., 2010). Long prenarial grooves located medial to the nares on the anterior margin of the cephalofoil funnel impinging water into the nares (Tester, 1963). This functional extension of the nares increases the effective sampling swath and potentially provides the shark with better spatial resolution for detecting odor cues (Abel et al., 2010; Kajiura et al., 2005; Tester, 1963). In contrast to sphyrnids, most other shark species, such as the pointed-snout carcharhinid sharks, possess nares that are positioned much closer together and lack prenarial grooves. Another morphological adaptation of the external olfactory structures is found in more benthically associated, sedentary species, including most batoid elasmobranchs (skates and rays). Similar to sedentary, demersal teleost species, modifications are necessary to promote irrigation of the olfactory capsule. Recall that in teleosts the beating cilia of the non-sensory cells generate water flow to help irrigate the olfactory organ; however, this mechanism is not employed by elasmobranchs (Theisen et al., 1986). A nasoral or naso-labial groove physically connects the mouth and the olfactory capsule, enabling them to flush water through the olfactory capsule using their buccopharyngeal respiratory pump similar to cyclosmate teleosts (Bell, 1993; Kleerekoper, 1969; Theisen et al., 1986). In addition to variations in the external morphology, elasmobranchs also exhibit interspecific differences in the morphology of their olfactory organs. The peripheral olfactory organ is comprised of numerous plate-like lamellae overlain with the OE that, in contrast to 8 teleosts, may exhibit some degree of secondary folding (Zeiske et al., 1986; Zeiske et al., 1987). The number of lamellae comprising the olfactory organ and the degree of secondary OE folding is highly variable among species (Kajiura et al., 2005; Meng and Yin, 1981; Meredith and Kajiura, 2010; Schluessel et al., 2008). In a survey that examined the olfactory morphology of 21 elasmobranch species, the number of lamellae ranged from 58 for Aptychotrema rostratato to 231 for Sphyrna lewini (Schluessel et al., 2008). Sphyrnid sharks typically possess more lamellae than other species due to their notably elongate olfactory organs; though this does not necessarily confer greater epithelial surface area (Kajiura et al., 2005). The surface area of the olfactory lamellae also differs among species, though the contribution of the secondary epithelial folds was not taken into account (Kajiura et al., 2005; Meredith and Kajiura, 2010; Schluessel et al., 2008). Schluessel and colleagues (2008) related these morphological factors to general habitat. They found that bentho-pelagic species possessed significantly more olfactory lamellae and larger epithelial surface areas than benthic species. Several studies suggest that species with larger olfactory structures (i.e. greater epithelial surface area or OB volume) should possess greater olfactory acuity or sensitivity (Lisney and Collin, 2006; Schluessel et al., 2008; Theiss et al., 2009). However, this idea has been refuted for teleosts, and a recent study on the olfactory morphology and physiology of five elasmobranch species found that olfactory sensitivity was neither correlated with lamellar counts nor lamellar surface area (Meredith and Kajiura, 2010). Similar to the OE ultrastructure of other vertebrates, the pseudostratified, columnar, sensory epithelium in elasmobranchs is populated with microvillous ORNs, ciliated supporting cells, and basal cells (Ferrando et al., 2006; Schluessel et al., 2008; Takami et al., 1994; Theisen et al., 1986; Zeiske et al., 1986). As in some teleost species, goblets cells are also present in the sensory OE (Schluessel et al., 2008; Takami et al., 1994) Although Bakhtin (1977) and Fishelson and Baranes (1997) reported that elasmobranch species also possess ciliated ORNs, the current consensus is that elasmobranchs lack the ciliated type of ORN found in teleosts and most other vertebrates (Bronshtein, 1976; Eisthen, 2004; Hansen et al., 2004; Reese and Brightman, 1970; Takami et al., 1994; Theisen et al., 1986; Zeiske et al., 1986). In 2006, crypt ORNs were 9 described in the small spotted catshark, Scyliorhinus canicula (Ferrando et al., 2006). This cell type was previously thought to be present only in the OE of teleost fishes (Hansen and Finger, 2000). In teleosts, the three ORN types each express a single molecular OR type coupled to a specific G-protein α subunit and are sensitive to a distinct suite of odorants (Hansen et al., 2003). The elasmobranch olfactory receptor gene expression is currently unknown, but research is beginning to uncover the G-protein expression in the OE. In a recent study, immunoreactivity to a particular Gα-protein subunit, Gαo, was detected in the OE of S. canicula (Ferrando et al., 2009). In the olfactory system of most vertebrates, this specific Gα-protein subunit is typically associated with microvillous ORNs, which occur in the elasmobranch OE (Hansen et al., 2004). Currently, the Gα-protein subunit coupled to crypt ORNs in elasmobranchs is unknown. The OB of elasmobranchs exhibits a pedunculated placement in contrast to the variable OB placement (pedunculated or sessile) of many teleost species (Northcutt, 1978; Zielinski and Hara, 2006). Similar to teleosts, the elasmobranch OB is also divided into four concentric layers: the olfactory nerve layer, the glomerular layer, the mitral cell layer, and the granule cell layer (Dryer and Graziadei, 1993; Ferrando et al., 2009; Takami et al., 1994; Tester, 1963). A superficial fibrous layer occurs in the posterior OB. At the anterior aspect of the OB, adjacent to the OB/OE interface, axons enter from the OE to form the olfactory nerve layer followed by a wide, ill-defined glomerular layer. In the glomerular layer of both the clearnose skate, Raja eglanteria, and the small-spotted catshark, Scyliorhinus canicula, mitral cells are interspersed among the glomeruli. Hence, these two layers (glomerular and mitral cell layers) are indistinguishable in contrast to the more distinct layers seen in certain teleost species (Baier and Korsching, 1994; Baier et al., 1994; Byrd and Brunjes, 1995; Ferrando et al., 2009; Satou, 1992; Takami et al., 1994). The deepest OB layer is the granule cell layer. Similar to teleosts, the olfactory tract connects the OB and remaining telencephalon and is divided into distinct medial and lateral parts projecting to separate telencephalic regions (Dryer and Graziadei, 1994b). Although the elasmobranch OB demonstrates many morphological similarities with the teleost OB, elasmobranchs may possess a larger olfactory brain area (Lisney and Collin, 2006). In a 10 comparison of the relative size and development of sensory brain areas in pelagic sharks and teleosts, shark species had larger OBs and were consequently hypothesized to be heavily reliant on smell, though this was not directly tested (Lisney and Collin, 2006). Some elasmobranch species, such as the lemon shark, Negaprion brevirostris, possess a unique OB morphology not documented in any other vertebrate (Northcutt, 1978). In these species, each OB is physically sub-divided into either a series of connected swellings or into two, distinct hemi-olfactory bulbs in contrast to the typical cohesive OB morphology seen in most vertebrates. The pervasiveness of this OB morphology in the subclass Elasmobranchii is unknown, but it is evident in the brain photographs and illustrations for several species represented in the works of Northcutt (1978) and Garman (1913). The functional significance of these hemi-OBs is not fully understood; however, it may reflect a segregation of olfactory input as seen in the teleost OB. Each half of the teleost OB, although physically cohesive, processes information on distinct odorant classes. The medial portion processes pheromonal information whereas the lateral portion processes non-pheromonal information, such as feeding cues (Hamdani and Døving, 2007; Nikonov and Caprio, 2001). An alternative explanation was put forth by Dryer and Graziadei (1993). They examined the OB organization of three elasmobranch species (Dasyatis sabina, Rhizoprionadon terranovae, and Sphyrna tiburo) and found that ORNs in the medial and lateral halves of the OE projected immediately posterior to the medial and lateral parts of the OB respectively. This suggests that ORN projections maintain their spatial organization from the OE to the OB, creating a somatotopic OB map that is seen in other sensory modalities, such as vision, in contrast to the chemotopic arrangement seen in the olfactory systems of teleost fishes and other vertebrates. A somatotopic organization of the elasmobranch OB would be unique among vertebrates, and further studies are needed to confirm this arrangement on a finer scale and determine how it affects the coding of olfactory information. Physiology of the teleost olfactory system As previously mentioned, ORN morphology is correlated to its specificity to odorants in teleosts. The range of odorants that stimulates the teleost olfactory system is quite different from 11 that detected by terrestrial vertebrates, likely due to differences in the presence of certain chemicals in the respective environments (Sorensen and Caprio, 1997). The olfactory system of fishes is sensitive to a variety of odorants, which include amino acids, polyamines, bile salts, gonadal steroids, prostaglandins, and nucleotides (Hara, 1994; Rolen et al., 2003; Zielinski and Hara, 2006). Physiological responses of fish to these odorants are commonly recorded using electro-olfactogram (EOG) or electro-encephalogram (EEG) techniques (Caprio, 1995; Ottoson, 1971; Silver et al., 1976). EOGs are the negative potential changes recorded in the water directly above the olfactory mucosa (over the OE) caused by the entry of cations into the activated ORNs during chemical stimulation. EEGs record rhythmic oscillatory neuron activity induced by chemical stimulation, usually in the OB or higher telencephalic regions. Physiological recordings facilitate the estimation of olfactory thresholds, comparisons of relative effectiveness among odorants, and distinction among putative receptor populations. These factors not only elucidate the olfactory capabilities of fishes, but also inform researchers of the biological relevance of different odorant classes. Amino acids, polyamines, and nucleotides are found in prey, stimulate feeding in teleost fishes, and thus are generally considered food-related odorants (Carr et al., 1996; Rolen et al., 2003; Sorensen and Caprio, 1997). These three types of compounds are potent olfactory stimuli -7 -9 to teleosts, with olfactory thresholds often estimated between 10 M to 10 M. Among the amino acids tested, L-amino acids with uncharged, unbranched side-chains are usually most stimulatory. Cross-adaptation and mixture experiments aim to determine whether two or more agonists interact with independent or overlapping OR populations. Results reveal multiple, partially independent amino acid receptor types capable of distinguishing among amino acids based on their side-chain structure. These include receptors for amino acids possessing sidechains that are acidic, basic, long and neutral, and short and neutral (Caprio and Byrd, 1984; Caprio et al., 1989; Kang and Caprio, 1991; Laberge and Hara, 2004). Cross-adaptation experiments with goldfish demonstrated that polyamine receptors are also independent from those for amino acids, nucleotides, and bile salts (Rolen et al., 2003). 12 Bile salts are biliary steroids produced by all vertebrates in the liver to emulsify lipids (Hagey et al., 2010; Haslewood, 1967; Hofmann et al., 2010). They are typically recycled by the enterohepatic system; however, agnathans and teleosts release a portion of their bile salts into the water via their urine or feces where they serve as potent olfactory stimuli (Velez et al., 2009; Zhang et al., 2001). Lampreys have been shown to use bile salts as pheromones to attract mates and guide adults to spawning streams (Li et al., 2002; Siefkes and Li, 2004; Sorensen et al., 2005). Bile salts have also been heavily implicated as pheromones for teleosts (Hara, 1994; Huertas et al., 2007; Sorensen and Caprio, 1997; Sorensen and Stacey, 2004; Zhang et al., 2001). For example, bile salts released by salmon may mediate the migration of adults to their natal streams (Døving et al., 1980). Due to the biological relevance of this odorant class, both teleosts and agnathans have demonstrated remarkable sensitivities to these compounds in the nanomolar range and lower and can distinguish among bile salts based on the type and position of the conjugating group (non-, taurine-, or glycine-conjugation) (Huertas et al., 2010; Li and Sorensen, 1997; Michel and Derbidge, 1997; Rolen and Caprio, 2007; Zhang and Hara, 2009). Between three and six bile salt ORs have been characterized for teleosts. Additionally, teleost fishes possess not only independent OR populations for bile salt and amino acid odorants, but also distinct ORN pathways into the central nervous system (Michel and Derbidge, 1997; Nikonov and Caprio, 2001; Zhang and Hara, 2009). It is currently unknown whether this also occurs in agnathans since research has revealed that they generally lack sensitivity to amino acids (Li and Sorensen, 1992). Gonadal steroids are hormones produced by the gonads that function as reproductive pheromones when released into the water via urine (Stacey and Sorensen, 2005). Steroids such as 17,20β-dihydroxy-4-pregnen-3-one (17,20β-P) stimulate oocyte maturation in female goldfish and are also released to the water to serve as a preovulatory pheromone, stimulating male reproductive behavior and sperm production (Kobayashi et al., 2002). Prostaglandins are a type of fatty acid that act as a hormone triggering female sexual behavior, and serve as pheromones for several teleost species (Sorensen and Goetz, 1993). For example, prostaglandin F 1a (PGF1α) 13 and PGF2α are excreted in the urine of mature ovulated female Atlantic salmon (Salmo salar) (Moore and Waring, 1996). Mature male salmon possess an increasing olfactory sensitivity to -11 PGF1α and PGF2α as the reproductive season progresses, with detection thresholds at 10 M. Exposure of male salmon to these PFGs results in a priming effect by inducing male spawning behavior and increasing sperm production (Kobayashi et al., 2002). Teleosts possess an acute olfactory sensitivity (in the picomolar range) and specificity to these compounds as they mediate one of the most important behaviors in the life of a fish (Sorensen and Caprio, 1997). Each of the different odorant classes are thought to be processed by distinct OB regions. Electro-physiological experiments confirmed the chemotopic organization of the teleost OB seen in axonal tracing and imaging studies by mapping bulbar neuron responses to various odorant classes across the OB. For example, in catfish, responses to bile salts were localized in the medial OB, responses to nucleotides occurred primarily in the dorsal, caudolateral OB, and amino acid responses were observed rostrally in the dorsolateral OB (Nikonov and Caprio, 2001). Data gathered from salmon and zebrafish generally conformed to a similar pattern of activity (Døving et al., 1980; Friedrich and Korsching, 1997; Friedrich and Korsching, 1998; Hara and Zhang, 1996; Hara and Zhang, 1997). The chemotopic organization of the OB, also found in other vertebrates, is thought to facilitate the discrimination and detection of odors and play an important role in quality coding of odorant information (Caprio and Derby, 2008; Hildebrand and Shepherd, 1997; Johnson and Leon, 2007; Nikonov and Caprio, 2001; Strausfeld and Hildebrand, 1999). Physiology of the elasmobranch olfactory system Elasmobranchs are widely considered to possess superior olfactory sensitivities compared to teleosts and are often referred to in the popular media as “swimming noses”. Their reputation is partly based on anecdotal observations, but is also due to the particularly large olfactory structures, including the epithelial surface area (Schluessel et al., 2008) and OB volume (Lisney et al., 2007; Lisney and Collin, 2006). Several studies relate the size of olfactory structures to the species ecology, invoking the assumption that differences in the size of olfactory structures correlate with olfactory sensitivity (Lisney et al., 2007; Lisney and Collin, 2006; 14 Northcutt, 1977; Northcutt, 1978; Schluessel et al., 2008; Theisen et al., 1986; Theiss et al., 2009; Yopak et al., 2007). This assumption has been refuted for teleost fishes (Hansen and Zielinski, 2005; Hara, 1994; Yamamoto, 1982) and recently for elasmobranchs as well (Meredith and Kajiura, 2010). Electrophysiological techniques were not utilized in olfactory studies with elasmobranchs until the mid-1960s (Gilbert et al., 1964). This research recorded EEGs from various locations in the brains of lemon sharks (Negaprion brevirostris), nurse sharks (Ginglymostoma cirratum), and bonnethead sharks (Sphyrna tiburo) and found the amplitude and frequency of the electric potentials increased during stimulation with food material, pure amino acids, and amines. This study was the first to demonstrate electrical correlates in the elasmobranch nervous system during chemical stimulation. Electrodes were also implanted in the OB and forebrain of freeswimming lemon and nurse sharks to allow correlation of brain potentials with behavioral responses during chemical stimulation (Hodgson and Mathewson, 1978). The strongest EEG and behavioral responses were elicited by amino acids and amines, similar to what had been observed with teleost fishes (Hara, 1994; Zielinski and Hara, 2001). An underwater EOG was also used to assess the responses of elasmobranchs to odorants (Silver et al., 1976); however only amino acids have been tested to date. The OE of elasmobranchs is highly sensitive to amino acid odorants, possibly due to the presence of -7 -9 microvillous ORNs, with olfactory thresholds estimated between 10 M to 10 M (Meredith and Kajiura, 2010; Silver, 1979; Tricas et al., 2009; Zeiske et al., 1986). Since elasmobranchs lack ciliated ORNs and the associated expression of Gαolf , it is unknown whether they, like teleosts, can detect bile salts. Though reputed to possess greater olfactory sensitivity than teleost fishes, olfactory thresholds estimated for several elasmobranch species to amino acids odorants are comparable to those estimated for teleosts (Hara, 1994; Meredith and Kajiura, 2010; Silver, 1979; Tricas et al., 2009; Zeiske et al., 1986). Since many fishes are subject to similar background amino acid levels in their environment, both teleosts and elasmobranchs appear to have converged upon similar amino acid sensitivities. Of the amino acid odorants assayed in these 15 studies, neutral L-amino acids such as alanine, serine, and methionine were most stimulatory. Neutral L-amino acids were previously demonstrated to be especially potent stimuli for teleost fishes as well (Caprio, 1978; Caprio, 1982; Hara, 1976; Hara, 1994). While popular belief alleges that elasmobranchs possess a more sensitive olfactory system than teleost fishes, empirical evidence is absent. The elasmobranch olfactory system is morphologically and functionally similar to that of teleost fishes in multiple ways. However, important differences exist between these two groups, emphasizing the need to test assumptions while taking evolutionary history into account. The olfactory capabilities of elasmobranchs have been explored preliminarily in morphological and physiological studies, but new integrative research techniques will elucidate the influence of ecology on their olfactory systems. An understanding of olfaction in this basal vertebrate group will also enhance our understanding of olfaction in more derived vertebrate clades. 16 REFERENCES Abel, R. L., Maclaine, J. S., Cotton, R., Xuan, V. B., Nickels, T. B., Clark, T. H., Wang, Z. and Cox, J. P. L. (2010). Functional morphology of the nasal region of a hammerhead shark. Comparative Biochemistry and Physiology - Part A: Molecular & Integrative Physiology 155, 464-475. Ache, B. W. and Young, J. M. (2005). Olfaction: Diverse Species, Conserved Principles. Neuron 48, 417-430. Baier, H. and Korsching, S. (1994). Olfactory Glomeruli in the Zebrafish Form an Invariant Pattern and Are Identifiable across Animals. J. Neurosci. 14, 219-230. Baier, H., Rotter, S. and Korsching, S. (1994). Connectional Topography in the Zebrafish Olfactory System - Random Positions but Regular Spacing of Sensory Neurons Projecting to an Individual Glomerulus. Proc. Natl. Acad. Sci. U. S. A. 91, 11646-11650. Bakhtin, E. K. (1977). Peculiarities of the fine structure of the olfactory organ of Squalus acanthius. Tsitologiya 14, 725-731. Belanger, R. M., Smith, C. M., Corkum, L. D. and Zielinski, B. S. (2003). Morphology and histochemistry of the peripheral olfactory organ in the round goby, Neogobius melanostomus (Teleostei : Gobiidae). J. Morphol. 257, 62-71. Bell, M. A. (1993). Convergent evolution of nasal structure in sedentary elasmobranchs. Copeia 1, 144-158. Bozza, T. C. and Kauer, J. S. (1998). Odorant response properties of convergent olfactory receptor neurons. J. Neurosci. 18, 4560-4569. Bronshtein, A. A. (1976). Some peculiarities of the fine structure of olfactory organ in elasmobranchs. ZH Evol Biokhim Fiz 12, 63-67. Buck, L. and Axel, R. (1991). A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell 65, 175-187. 17 Byrd, C. A. and Brunjes, P. C. (1995). Organization of the Olfactory System in the Adult Zebrafish - Histological, Immunohistochemical, and Quantitative-Analysis. J. Comp. Neurol. 358, 247-259. Caprio, J. (1978). Olfaction and Taste in Channel Catfish - Electrophysiological Study of Responses to Amino-Acids and Derivatives. Journal of Comparative Physiology 123, 357-371. Caprio, J. (1982). High sensitivity and specificity of olfactory and gustatory receptors of catfish to amino acids. In Chemoreception in Fishes, (ed. T. J. Hara), pp. 109-134. Amsterdam: Elsevier. Caprio, J. (1995). In vivo olfactory and taste recordings in fish. In Experimental cell biology of taste and olfaction, eds. A. I. Spielman and J. G. Brand), pp. 251-261. Boca Raton: CRC Press. Caprio, J. and Byrd, R. P. (1984). Electrophysiological Evidence for Acidic, Basic, and Neutral Amino-Acid Olfactory Receptor-Sites in the Catfish. J. Gen. Physiol. 84, 403-422. Caprio, J. and Derby, C. D. (2008). Aquatic animal models in the study of chemoreception. In The Senses: A Comprehensive Reference, vol. 4: Olfaction and Taste eds. A. I. Basbaum A. Kaneko G. M. Shepherd and G. Westheimer), pp. 97-134. San Diego, CA: Academic Press. Caprio, J., Dudek, J. and Robinson, J. J. (1989). Electro-Olfactogram and Multiunit Olfactory Receptor Responses to Binary and Trinary Mixtures of Amino-Acids in the Channel Catfish, Ictalurus-Punctatus. J. Gen. Physiol. 93, 245-262. Carr, W., Netherton JC, I., Gleeson, R. A. and Derby, C. D. (1996). Stimulants of Feeding Behavior in Fish: Analyses of Tissues of Diverse Marine Organisms. Biol. Bull. 190, 149160. Carr, W. E. S., Trapido-Rosenthal, H. G. and Gleeson, R. A. (1989). Stimulants of feeding behavior in marine organisms: receptors and perireceptor events provide insight into mechanisms of mixture interaction. Perception of Complex Smells and Tastes, 27-45. 18 Cox, J. P. L. (2008). Hydrodynamic aspects of fish olfaction. J. R. Soc. Interface 5, 575-593. Døving, K. B., Duboisdauphin, M., Holley, A. and Jourdan, F. (1977). Functional Anatomy of Olfactory Organ of Fish and Ciliary Mechanism of Water Transport. Acta Zool. 58, 245255. Døving, K. B., Hansson, K. A., Backstrom, T. and Hamdani, E. H. (2011). Visualizing a set of olfactory sensory neurons responding to a bile salt. J. Exp. Biol. 214, 80-87. Døving, K. B. and Selset, R. (1980). Behavior Patterns in Cod Released by Electrical-Stimulation of Olfactory Tract Bundlets. Science 207, 559-560. Døving, K. B., Selset, R. and Thommesen, G. (1980). Olfactory Sensitivity to Bile-Acids in Salmonid Fishes. Acta Physiol. Scand. 108, 123-131. Dryer, L. and Graziadei, P. P. C. (1993). A Pilot-Study on Morphological Compartmentalization and Heterogeneity in the Elasmobranch Olfactory-Bulb. Anat. Embryol. (Berl). 188, 4151. Dryer, L. and Graziadei, P. P. C. (1994a). Mitral Cell Dendrites - a Comparative Approach. Anat. Embryol. (Berl). 189, 91-106. Dryer, L. and Graziadei, P. P. C. (1994b). Projections of the Olfactory Bulb in an Elasmobranch Fish, Sphyrna tiburo: Segregation of Inputs in the Telencephalon. Anat. Embryol. (Berl). 190, 563-572. Edrén, S. M. C. and Gruber, S. H. (2005). Homing ability of young lemon sharks, Negaprion brevirostris. Environ. Biol. Fishes 72, 267-281. Eisthen, H. L. (2002). Why Are Olfactory Systems of Different Animals So Similar? Brain, Behavior & Evolution 59, 273. Eisthen, H. L. (2004). The goldfish knows: Olfactory receptor cell morphology predicts receptor gene expression. J. Comp. Neurol. 477, 341-346. Ferrando, S., Bottaro, M., Gallus, L., Girosi, L., Vacchi, M. and Tagliafierro, G. (2006). Observations of crypt neuron-like cells in the olfactory epithelium of a cartilaginous fish. Neurosci. Lett. 403, 280-282. 19 Ferrando, S., Gambardella, C., Ravera, S., Bottero, S., Ferrando, T., Gallus, L., Manno, V., Salati, A. P., Ramoino, P. and Tagliafierro, G. (2009). Immunolocalization of G-Protein Alpha Subunits in the Olfactory System of the Cartilaginous Fish Scyliorhinus Canicula. Anatomical Record-Advances in Integrative Anatomy and Evolutionary Biology 292, 1771-1779. Firestein, S. (2001). How the olfactory system makes sense of scents. Nature 413, 218. Fishelson, L. and Baranes, A. (1997). Ontogenesis and cytomorphology of the nasal olfactory organs in the Oman shark, Iago omanensis (Triakidae), in the Gulf of Aqaba, Red Sea. Anat. Rec. 249, 409-421. Friedrich, R. W. and Korsching, S. I. (1997). Combinatorial and Chemotopic Odorant Coding in the Zebrafish Olfactory Bulb Visualized by Optical Imaging. Neuron 18, 737-752. Friedrich, R. W. and Korsching, S. I. (1998). Chemotopic, Combinatorial, and Noncombinatorial Odorant Representations in the Olfactory Bulb Revealed Using a Voltage-Sensitive Axon Tracer. J. Neurosci. 18, 9977-9988. Garman, S. (1913). The Plagiostomia (sharks, skates, and rays). Cambridge, MA: Printed for the museum. Gilbert, P. W., Hodgson, E. S. and Mathewson, R. F. (1964). Electroencephalograms of sharks. Science 145, 949-951. Hagey, L. R., MØller, P. R., Hofmann, A. F. and Krasowski, M. D. (2010). Diversity of Bile Salts in Fish and Amphibians: Evolution of a Complex Biochemical Pathway. Physiol. Biochem. Zool. 83, 308-321. Hamdani, E. H. and Døving, K. B. (2007). The functional organization of the fish olfactory system. Prog. Neurobiol. 82, 80-86. Hamdani, E. H., Kasumyan, A. and Døving, K. B. (2001). Is feeding behaviour in crucian carp mediated by the lateral olfactory tract? Chem. Senses 26, 1133-1138. 20 Hamdani, E. H., Stabell, O. B., Alexander, G. and Døving, K. B. (2000). Alarm reaction in the crucian carp is mediated by the medial bundle of the medial olfactory tract. Chem. Senses 25, 103-109. Hansen, A., Anderson, K. T. and Finger, T. E. (2004). Differential distribution of olfactory receptor neurons in goldfish: Structural and molecular correlates. J. Comp. Neurol. 477, 347-359. Hansen, A. and Finger, T. E. (2000). Phyletic distribution of crypt-type olfactory receptor neurons in fishes. Brain. Behav. Evol. 55, 100-10. Hansen, A., Rolen, S. H., Anderson, K., Morita, Y., Caprio, J. and Finger, T. E. (2003). Correlation between Olfactory Receptor Cell Type and Function in the Channel Catfish. The Journal of Neuroscience 23, 9328-9339. Hansen, A. and Zeiske, E. (1998). The peripheral olfactory organ of the zebrafish, Danio rerio: an ultrastructural study. Chem. Senses 23, 39-48. Hansen, A. and Zielinski, B. (2005). Diversity in the olfactory epithelium of bony fishes: Development, lamellar arrangement, sensory neuron cell types and transduction components. J. Neurocytol. 34, 183-208. Hara, T. J. (1976). Structure-activity relationships of amino acids in fish olfaction. Comparative Biochemistry and Physiology Part A: Physiology 54, 31-36. Hara, T. J. (1994). The Diversity of Chemical Stimulation in Fish Olfaction and Gustation. Rev. Fish Biol. Fisher. 4, 1-35. Hara, T. J. and Zhang, C. (1996). Spatial projections to the olfactory bulb of functionally distinct and randomly distributed primary neurons in salmonid fishes. Neurosci. Res. 26, 65-74. Hara, T. J. and Zhang, C. (1997). Topographic bulbar projections and dual neural pathways of the primary olfactory neurons in salmonid fishes. Neuroscience 82, 313. Haslewood, G. A. D. (1967). Bile salt evolution. J. Lipid Res. 8, 535-550. Hildebrand, J. G. and Shepherd, G. M. (1997). Mechanisms of olfactory discrimination: Converging evidence for common principles across phyla. Annu. Rev. Neurosci. 20, 595631. 21 Hodgson, E. S. and Mathewson, R. F. (1978). Electrophysiological studies of chemoreception in elasmobranchs. In Sensory biology of sharks, skates and rays, eds. E. S. Hodgson and R. F. Mathewson), pp. 227-266. Washington D.C.: Government Printing Office. Hodos, W. and Butler, A. B. (1997). Evolution of sensory pathways in vertebrates. Brain Behav. Evolut. 50, 189-197. Hofmann, A. F., Hagey, L. R. and Krasowski, M. D. (2010). Bile salts of vertebrates: structural variation and possible evolutionary significance. J. Lipid Res. 51, 226-246. Huertas, M., Hagey, L., Hofmann, A. F., Cerda, J., Canario, A. V. M. and Hubbard, P. C. (2010). Olfactory sensitivity to bile fluid and bile salts in the European eel (Anguilla anguilla), goldfish (Carassius auratus) and Mozambique tilapia (Oreochromis mossambicus) suggests a 'broad range' sensitivity not confined to those produced by conspecifics alone. J. Exp. Biol. 213, 308-317. Huertas, M., Hubbard, P. C., Canario, A. V. M. and Cerda, J. (2007). Olfactory sensitivity to conspecific bile fluid and skin mucus in the European eel Anguilla anguilla (L.). J Fish Biol 70, 1907-1920. Hueter, R. E., Mann, D. A., Maruska, K. P., Sisneros, J. A. and Demski, L. S. (2004). Sensory Biology of Elasmobranchs. In Biology of Sharks and Their Relatives, eds. J. C. Carrier J. A. Musick and M. R. Heithaus). Boca Raton, London, New York, Washington D.C.: CRC Press. Janvier, P. (1996). Early vertebrates. Oxford, England: Oxford University Press. Johnson, B. A. and Leon, M. (2007). Chemotopic odorant coding in a mammalian olfactory system. J. Comp. Neurol. 503, 1-34. Johnson, R. H. and Nelson, D. R. (1978). Copulation and possible olfaction-mediated pair formation in two species of carcharhinid sharks. Copeia 3, 539-542. Kajiura, S. M., Forni, J. B. and Summers, A. P. (2005). Olfactory morphology of carcharhinid and sphyrnid sharks: Does the cephalofoil confer a sensory advantage? J. Morphol. 264, 253263. 22 Kajiura, S. M., Sebastian, A. P. and Tricas, T. C. (2000). Dermal Bite Wounds as Indicators of Reproductive Seasonality and Behaviour in the Atlantic Stingray, Dasyatis sabina. Environ. Biol. Fishes 58, 23-31. Kang, J. S. and Caprio, J. (1991). Electro-Olfactogram and Multiunit Olfactory Receptor Responses to Complex-Mixtures of Amino-Acids in the Channel Catfish, IctalurusPunctatus. J. Gen. Physiol. 98, 699-721. Kleerekoper, H. (1969). Olfaction in fishes. Bloomington: Indiana University Press. Kleerekoper, H. (1978). Chemoreception and the role of its interaction with flow and light perception in the locomotion and orientation of some elasmobranchs. In Sensory biology of shark, skates, and rays, eds. E. S. Hodgson and R. F. Mathewson), pp. 269-329. Washington, D. C.: Government Printing Office. Kobayashi, M., Sorensen, P. W. and Stacey, N. E. (2002). Hormonal and pheromonal control of spawning behavior in the goldfish. Fish Physiol. Biochem. 26, 71-84. Laberge, F. and Hara, T. J. (2001). Neurobiology of fish olfaction: a review. Brain Research Reviews 36, 46-59. Laberge, F. and Hara, T. J. (2004). Electrophysiological demonstration of independent olfactory receptor types and associated neuronal responses in the trout olfactory bulb. Comparative Biochemistry and Physiology - Part A: Molecular & Integrative Physiology 137, 397-408. Laframboise, A. J., Ren, X., Chang, S., Dubuc, R. and Zielinski, B. S. (2007). Olfactory sensory neurons in the sea lamprey display polymorphisms. Neurosci. Lett. 414, 277-281. Li, W., Scott, A. P., Siefkes, M. J., Yan, H., Liu, Q., Yun, S.-S. and Gage, D. A. (2002). Bile Acid Secreted by Male Sea Lamprey That Acts as a Sex Pheromone. Science 296, 138. Li, W. and Sorensen, P. W. (1992). The olfactory sensitivity of sea lamprey to amino acids is specifically resricted to arginine. Chem. Senses 17, 658. 23 Li, W. and Sorensen, P. W. (1997). Highly independent olfactory receptor sites for naturally occurring bile acids in the sea lamprey, Petromyzon marinus. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology 180, 429-438. Lim, D. D., Motta, P., Mara, K. and Martin, A. P. (2010). Phylogeny of hammerhead sharks (Family Sphyrnidae) inferred from mitochondrial and nuclear genes. Mol. Phylogenet. Evol. 55, 579. Lisney, T. J. and Collin, S. P. (2006). Brain morphology in large pelagic fishes: a comparison between sharks and teleosts. J Fish Biol 68, 532-554. Meng, Q. and Yin, M. (1981). A study of the olfactory organ of the shark. Transactions of the Chinese Ichthyological Society 2, 1-24. Meredith, T. L. and Kajiura, S. M. (2010). Olfactory morphology and physiology of elasmobranchs. Journal of Experimental Biology 213, 3449-3456. Michel, W. C. and Derbidge, D. S. (1997). Evidence of distinct amino acid and bile salt receptors in the olfactory system of the zebrafish, Danio rerio. Brain Res. 764, 179-187. Moore, A. and Waring, C. P. (1996). Electrophysiological and endocrinological evidence that Fseries prostaglandins function as priming pheromones in mature male Atlantic salmon (Salmo salar) Parr. J. Exp. Biol. 199, 2307-2316. Morita, Y. and Finger, T. E. (1998). Differential projections of ciliated and microvillous olfactory receptor cells in the catfish, Ictalurus punctatus. The Journal of Comparative Neurology 398, 539-550. Moyle, P. B. and Cech, J. J. (2004). Fishes: An Introduction to Ichthyology. Upper Saddle River, NJ: Prentice Hall. Nevitt, G. A. (1991). Do Fish Sniff - a New Mechanism of Olfactory Sampling in Pleuronectid Flounders. J. Exp. Biol. 157, 1-18. Nikonov, A. A. and Caprio, J. (2001). Electrophysiological evidence for a chemotopy of biologically relevant odors in the olfactory bulb of the channel catfish. J. Neurophysiol. 86, 1869-1876. 24 Northcutt, R. G. (1978). Brain organization in the cartilagenous fishes. In Sensory biology of shark, skates, and rays, eds. E. S. Hodgson and R. F. Mathewson), pp. 117-193. Washington, D. C.: Government Printing Press. Oka, Y., Ichikawa, M. and Ueda, K. (1982). Synaptic organization of the olfactory bulb and central projection of the olfactory tract. In Chemoreception in Fishes, (ed. T. J. Hara), pp. 61-76. Amsterdam: Elsevier. Ottoson, D. (1971). The electro-olfactogram. In Handbook of Sensory Physiology: Olfaction, vol. IV, Part 1 (ed. L. M. Beidler), pp. 95-131. Berlin, Heidelberg, New York: Springer-Verlag. Parker, G. H. (1913). The directive influence of the sense of smell in the dogfish. In Bulletin of the United States Bureau of Fisheries, vol. 33 (ed. H. M. Smith, Commissioner), pp. 63-68. Washington, D. C.: Government Printing Office. Rasmussen, L. E. L. and Schmidt, M. J. (1992). Are sharks chemically aware of crocodiles? In Chemical Signals in Vertebrates VI, vol. VI eds. R. L. Doty and D. Muller-Schwarze), pp. 335-341. New York: Plenum Press. Reese, T. S. and Brightman, M. W. (1970). Olfactory surfcace and central olfactory connexions in some vertebrates. In Taste and smell in vertebrate, eds. G. E. W. Wolstenholme and J. Knight), pp. 115-149. London: J. & A. Churchill. Rolen, S. H. and Caprio, J. (2007). Processing of bile salt odor information by single olfactory bulb neurons in the channel catfish. J. Neurophysiol. 97, 4058-4068. Rolen, S. H., Sorensen, P. W., Mattson, D. and Caprio, J. (2003). Polyamines as olfactory stimuli in the goldfish Carassius auratus. J. Exp. Biol. 206, 1683-1696. Sato, Y., Miyasaka, N. and Yoshihara, Y. (2005). Mutually exclusive glomerular innervation by two distinct types of olfactory sensory neurons revealed in transgenic zebrafish. J. Neurosci. 25, 4889-4897. Satou, M. (1992). Synaptic organization of the olfactory bulb and its central projection. In Fish Chemoreception, (ed. T. J. Hara), pp. 40-59. London: Chapman & Hall. 25 Schluessel, V., Bennett, M. B., Bleckmann, H., Blomberg, S. and Collin, S. P. (2008). Morphometric and ultrastructural comparison of the olfactory system in elasmobranchs: The significance of structure-function relationships based on phylogeny and ecology. J. Morphol. 269, 1365-1386. Sheldon, R. E. (1909). The reactions of the dogfish to chemical stimuli. J Comp. Neurol. Psycho. 19, 273-311. Sheldon, R. E. (1911). The sense of smell in Selachians. J. Exp. Zool. 10, 51-62. Siefkes, M. J. and Li, W. (2004). Electrophysiological evidence for detection and discrimination of pheromonal bile acids by the olfactory epithelium of female sea lampreys (Petromyzon marinus). J. Comp. Physiol. A -Neuroethol. Sens. Neural Behav. Physiol. 190, 193-199. Silver, W. L. (1979). Olfactory Responses from a Marine Elasmobranch, the Atlantic Stingray, Dasyatis sabina. Mar. Behav. Physiol. 6, 297-305. Silver, W. L., Caprio, J., Blackwell, J. F. and Tucker, D. (1976). Underwater Electro-Olfactogram Tool for Study of Sense of Smell of Marine Fishes. Experientia 32, 1216-1217. Smith, B. R. and Tibbles, J. J. (1980). Sea Lamprey (Petromyzon marinus) in Lakes Huron, Michigan, and Superior: History of Invasion and Control, 1936-78. Can. J. Fish. Aquat. Sci. 37, 1780-1801. Sorensen, P. W. and Caprio, J. (1997). Chemoreception. In The Physiology of Fishes, (ed. D. H. Evans), pp. 375-405. Boca Raton, FL: CRC Press. Sorensen, P. W., Fine, J. M., Dvornikovs, V., Jeffrey, C. S., Shao, F., Wang, J. Z., Vrieze, L. A., Anderson, K. R. and Hoye, T. R. (2005). Mixture of new sulfated steroids functions as a migratory pheromone in the sea lamprey. Nat. Chem. Biol. 1, 324-328. Sorensen, P. W. and Goetz, F. W. (1993). Pheromonal and Reproductive Function of FProstaglandins and Their Metabolites in Teleost Fish. J. Lipid Mediat. 6, 385-393. Sorensen, P. W. and Stacey, N. E. (2004). Brief review of fish pheromones and discussion of their possible uses in the control of non-indigenous teleost fishes. N. Z. J. Mar. Freshw. Res. 38, 399-417. 26 Stacey, N. E. and Sorensen, P. W. (2005). Hormones, pheromones, and reproductive behaviors. In Behaviour: Interactions with Fish Physiology, vol. 24 eds. K. A. Sloman S. Balshine and R. W. Wilson), pp. 359-412. New York, NY: Academic Press. Strausfeld, N. J. and Hildebrand, J. G. (1999). Olfactory systems: common design, uncommon origins? Curr. Opin. Neurobiol. 9, 634-639. Takami, S., Luer, C. A. and Graziadei, P. C. (1994). Microscopic structre of the olfactory organ of the clearnose skate, Raja eglanteria. Anat. Embryol. (Berl). 190, 211-230. Tester, A. L. (1963). Olfaction, gustation and the common chemical sense in sharks. In Sharks and Survival, (ed. P. W. Gilbert), pp. 243-254. Boston, MA: DC Health. Theisen, B., Zeiske, E. and Breucker, H. (1986). Functional morphology of the olfactory organs in the spiny dogfish (Squalus acanthius L.) and the small-spotted catshark (Scyliorhinus canicula (L.)). Acta Zool. 67, 73-86. Theiss, S. M., Hart, N. S. and Collin, S. P. (2009). Morphological Indicators of Olfactory Capability in Wobbegong Sharks (Orectolobidae, Elasmobranchii). Brain Behav. Evolut. 73, 91-101. Tricas, T. C., Kajiura, S. M. and Summers, A. P. (2009). Response of the hammerhead shark olfactory epithelium to amino acid stimuli. J. Comp. Physiol. A 195, 947-954. Velez, Z., Hubbard, P. C., Welham, K., Hardege, J. D., Barata, E. N. and Canario, A. V. M. (2009). Identification, release and olfactory detection of bile salts in the intestinal fluid of the Senegalese sole (Solea senegalensis). J. Comp. Physiol. A -Neuroethol. Sens. Neural Behav. Physiol. 195, 691-698. Weltzien, F. A., Hoglund, E., Hamdani, E. H. and Døving, K. B. (2003). Does the lateral bundle of the medial olfactory tract mediate reproductive behavior in male crucian carp? Chem. Senses 28, 293-300. Xu, F. Q., Greer, C. A. and Shepherd, G. M. (2000). Odor maps in the olfactory bulb. J. Comp. Neurol. 422, 489-495. Yamamoto, M. (1982). Comparative morphology of the peripheral olfactory organ in teleosts. In Chemoreception in fishes, (ed. T. J. Hara), pp. 39-59. New York, NY: Elsevier. 27 Zeiske, E., Caprio, J. and Gruber, S. H. (1986). Morphological and electrophysiological studies on the olfactory organ of the lemon shark, Negaprion brevirostris. In Indo-Pacific Fish Biology: Proceedings of the Second International Conference on Indo-Pacific Fishes, eds. T. Uyeno R. Arai T. Taniuchi and K. Matsuura), pp. 381-391. Tokyo: Ichtyological Society of Japan. Zeiske, E., Kasumyan, A., Bartsch, P. and Hansen, A. (2003). Early development of the olfactory organ in sturgeons of the genus Acipenser: a comparative and electron microscopic study. Anat. Embryol. (Berl). 206, 357-372. Zeiske, E., Theisen, B. and Gruber, S. H. (1987). Functional morphology of the olfactory organ of two carcharhinid shark species. Can. J. Zool. 65. Zhang, C. and Hara, T. J. (2009). Lake char (Salvelinus namaycush) olfactory neurons are highly sensitive and specific to bile acids. J. Comp. Physiol. A -Neuroethol. Sens. Neural Behav. Physiol. 195, 203-215. Zhang, C. B., Brown, S. B. and Hara, T. J. (2001). Biochemical and physiological evidence that bile acids produced and released by lake char (Salvelinus namaycush) function as chemical signals. J. Comp. Physiol. B-Biochem. Syst. Environ. Physiol. 171, 161-171. Zielinski, B. and Hara, T. J. (2001). The Neurobiology of Fish Olfaction. In Sensory Biology of Jawed Fishes: New Insights, eds. B. G. Kapoor and T. J. Hara), pp. 347-366. Enfield, NH: Science Publishers, Inc. Zielinski, B. and Hara, T. J. (2006). Olfaction. In Fish Physiology: Sensory Systems Neuroscience, vol. 25 eds. B. Zielinski and T. J. Hara), pp. 1-43: Academic Press. 28 CHAPTER 2 OLFACTORY MORPHOLOGY AND PHYSIOLOGY OF ELASMOBRANCHS ABSTRACT Elasmobranch fishes are thought to possess greater olfactory sensitivities than teleost fishes due in part to the large amount of epithelial surface area that comprises their olfactory organs; however, direct evidence correlating the size of the olfactory organ to olfactory sensitivity is lacking. This study examined the olfactory morphology and physiology of five distantly related elasmobranch species. Specifically, we quantified the number of lamellae and lamellar surface area (as if it were a flat sheet not considering secondary lamellae) that comprise their olfactory organs. We also calculated the olfactory thresholds and relative effectiveness of amino acid odorants for each species. The olfactory organs varied in both the number of lamellae and lamellar surface area, which may be related to their general habitat, but neither correlated with olfactory threshold. Thresholds to amino acid odorants, major olfactory stimuli of all fishes, ranged from 10 -9.0 -6.9 to 10 M; however, our results indicate that these elasmobranch species demonstrate comparable thresholds to teleosts. In addition, the relative effectiveness of amino acid stimuli to the olfactory organ of elasmobranchs is similar to that previously described in teleosts with neutral amino acids eliciting significantly greater responses than others. Collectively, these results indicate parallels in olfactory physiology between these two groups of fishes. INTRODUCTION Elasmobranch fishes (sharks, skates, and rays) are widely considered to possess superior olfactory sensitivities compared to bony fishes and are often referred to in the popular media as “swimming noses”. This reputation is partly based on anecdotal observations, but is 29 also due to their particularly large olfactory structures, including olfactory epithelial surface area (Schluessel et al., 2008) and olfactory bulb volume (Lisney et al., 2007; Lisney and Collin, 2006). Several studies related the size of their olfactory structures to species ecology, invoking the assumption that differences in the size of olfactory structures correlate with olfactory sensitivity (Lisney et al., 2007; Lisney and Collin, 2006; Northcutt, 1977; Northcutt, 1978; Schluessel et al., 2008; Theisen et al., 1986; Theiss et al., 2009; Yopak et al., 2007). This assumption has been refuted for teleost fishes (Hansen and Zielinski, 2005; Hara, 1994; Yamamoto, 1982) and has yet to be tested for elasmobranchs. To determine whether a correlation exists between olfactory organ (rosette) size and olfactory sensitivity requires a quantitative comparison of the olfactory morphology and response thresholds for multiple elasmobranch species. Comparative morphological studies demonstrated that elasmobranchs, like teleost fishes, exhibit interspecific differences in the number and surface area of olfactory lamellae (Hansen and Zielinski, 2005; Kajiura et al., 2005; Schluessel et al., 2008; Theiss et al., 2009; Yamamoto, 1982). Whereas the gross morphology and ultrastructure of the elasmobranch olfactory system are well described (Bell, 1993; Bronshtein, 1976; Meng and Yin, 1981; Schluessel et al., 2008; Tester, 1963; Theisen et al., 1986; Zeiske et al., 1986), olfactory thresholds have been assessed for only five elasmobranch species: the nurse shark, (Hodgson and Mathewson, 1978); the Atlantic stingray, Dasyatis sabina (Silver, 1979); lemon shark, Negaprion brevirostris (Zeiske et al., 1986); black sea skate, Raja clavata (Nikonov et al., 1990); and scalloped hammerhead shark, Sphyrna lewini (Tricas et al., 2009). Although evidence is limited, the threshold ranges of these species for amino acid stimuli are similar to those estimated for teleosts (Hara, 1994); however interspecific comparisons of the olfactory capabilities among multiple elasmobranch species are lacking. Only two of these studies integrated olfactory morphology with physiology, each on a single species (Silver, 1979; Zeiske et al., 1986), which precluded the ability to determine whether the size of the olfactory structures correlated with the estimated olfactory thresholds. 30 This study addresses long standing assumptions about elasmobranch olfaction by correlating the olfactory morphology and physiology of five phylogenetically diverse elasmobranch species. Specifically, we tested: 1) whether the lamellar surface area of the elasmobranch olfactory organ is correlated with olfactory threshold; 2) whether distantly related species demonstrate differences in olfactory threshold; and 3) whether elasmobranch fishes possess lower olfactory thresholds than teleost fishes. MATERIALS AND METHODS Animal Collection The olfactory system was examined in five elasmobranch species: the clearnose skate, Raja eglanteria Bosc 1800; yellow stingray, Urobatis jamaicensis (Cuvier 1816); Atlantic stingray, D. sabina (Lesueur 1824); lemon shark, N. brevirostris (Poey 1868); and bonnethead shark, Sphyrna tiburo (Linnaeus 1758). Raja eglanteria were acquired from a captive breeding population at the Mote Marine Laboratory (Sarasota, FL, USA). The other four species were collected from Florida near shore waters using long-lining, seining and hand-netting techniques. All animals used in electro-physiology experiments, except for S. tiburo, were transported to the Florida Atlantic University Marine Lab at the Gumbo Limbo Environmental Complex (Boca Raton, FL), maintained in tanks with flow-through seawater, and fed a diet of shrimp and squid daily to satiation. Sphyrna tiburo were maintained at Mote Marine Laboratory and fed daily to satiation until tested at that facility. All experiments were conducted in accordance with approved IACUC protocols at both Florida Atlantic University (A08-05) and Mote Marine Laboratory (09-10-SK2). Morphology The elasmobranch olfactory organs (rosettes) are comprised of numerous plate-like lamellae which are overlain with an olfactory epithelium and populated with olfactory receptor neurons (ORNs) (Theisen et al., 1986; Zeiske et al., 1986). The number of lamellae and total lamellar surface areas were quantified for a minimum of nine individuals for each of the five species. The lamellae were counted from a single olfactory organ from each animal. The number of lamellae was compared among the five species using a one-way analysis of variance 31 (ANOVA) followed by Tukey post hoc tests for pair-wise comparisons. We also pooled the lamellar counts for the sharks (bentho-pelagic) and the batoids (benthic) in order to compare the number of lamellae between habitats using a Mann-Whitney rank sum test (Systat Software, Inc., San Jose, CA, USA). To quantify the lamellar surface area, a representative subset of ten lamellae was dissected from one olfactory organ of each animal. This included the first, last, and eight intermediate lamellae evenly spaced along the length of the organ. Each lamella was digitally photographed on a micrometer slide to provide scale, and the surface area was measured using the software ImageJ, (National Institutes of Health, Bethesda, MA, USA). A quadratic equation was then fit to the ten surface area measurements, and a Simpson‟s rule numerical integration was applied in order to approximate the total lamellar surface area for each organ. The total lamellar surface area was then quadrupled to account for the presence of the olfactory epithelium on both sides of each lamella in two organs. The mean total lamellar surface area for each species was compared using an analysis of covariance (ANCOVA) (SPSS Inc., Chicago, IL, USA) with disc width (DW) for the batoids and total length (TL) for the sharks as the covariate, followed by LSD (least significant difference) post hoc tests for pair-wise comparisons. Body mass was not used as the covariate since this measurement was not available for all specimens; thus, surface area comparisons were not made between batoids and sharks due to differing body size metrics. Electrophysiology: Experimental Apparatus The underwater electro-olfactogram (EOG), an odorant-induced, slow negative potential thought to reflect summated olfactory receptor generator potentials, was measured in the water immediately above the olfactory organ (Caprio, 1995; Silver et al., 1976). The experimental apparatus consisted of an electrically grounded acrylic experimental tank (89x43x21 cm) supplied with flow-through seawater, which was mechanically (25 m polyscreen) and chemically (activated charcoal) filtered (Fig. 2.1). Seawater was delivered to the tank through three arms of a PVC manifold with the flow for each arm controlled by a ball or gate valve. One arm provided 32 ventilatory water flow over the gills. Seawater flow through the tank was provided via a second manifold arm and continuously drained to reduce chemical accumulation. A third arm of the manifold delivered a constant flow of seawater through a flow meter to paired computercontrolled, three-way solenoid valves. The first valve diverted the water flow to either of two short lengths of tubing (left or right branches) which both connected to the second solenoid valve. This second valve directed the flow from either branch to an odor delivery pipette positioned in the animal‟s incurrent naris, providing a constant flow of seawater over the olfactory organ. Seawater flowed through only one branch at a time, which enabled the injection of a stimulus into the inactive branch without disturbing the water flow over the olfactory organ. A remotely operated trigger enabled the solenoid valves to divert the water flow to deliver the stimulus bolus to the olfactory organ. This eliminated pressure artifacts in the EOG trace during stimulus injection. To record an animal‟s responses to odor stimuli, a non-polarizable Ag-AgCl electrode (E45P-M15NH, Warner Instruments, Hamden, CT, USA) fitted with a seawater/agar-filled capillary tube was positioned just above the olfactory epithelium and a similar reference electrode was placed nearby in contact with the animal‟s skin. The output from the two electrodes was differentially amplified (DP-304, Warner Instruments) at 1000-10000x, filtered (0.1 Hz - 0.1 kHz, 50/60 Hz) (DP-304, Warner Instruments & Hum Bug, Quest Scientific, North Vancouver, BC, CA), digitized at 1 kHz using a Power Lab® 16/30 model ML 880 (AD Instruments, Colorado Springs, CO, USA) and recorded using Chart™ Software (AD Instruments). Electrophysiology: Experimental Protocol Our experimental procedures closely followed previous EOG studies with elasmobranchs (Silver, 1979; Tricas et al., 2009; Zeiske et al., 1986). Prior to experimentation, an animal was injected (intramuscularly or intravenously) with the paralytic, pancuronium bromide (0.03 mg/kg). Immediately upon cessation of active ventilation the animal was transferred to the experimental ® tank, secured ventral side up with Velcro straps to a submerged platform, and ventilated with seawater via the mouth (sharks) or spiracles (batoids). For the batoids, a small sponge was fitted into the mouth to direct water flow from the spiracles over the gills. The odor delivery pipette was 33 mounted in a micromanipulator, and the pipette tip was positioned in the incurrent naris with -1 water flow regulated to 2 ml s (Tricas et al., 2009). A test dye solution was delivered through the odor delivery pipette to confirm that seawater flowed over the olfactory epithelium and exited the excurrent naris. The EOG electrodes were mounted in micromanipulators with the recording electrode positioned into the excurrent naris just above the olfactory epithelium and the reference electrode positioned nearby in contact with the skin. Appropriate placement of the recording electrode in each animal was confirmed by observing an adequately sized (minimum of 30 µV) -3 response to a 1.0 ml injection of 10 M alanine (standard). The heart beat was monitored with an EKG (sharks) or visually (batoids) throughout the experiments. The olfactory responses to twenty L-amino acids applied individually were recorded from -1 each animal. Amino acid stock solutions (10 M) were prepared with filtered seawater weekly and stored at 4ºC (pH 7.1 - 8.4). Dilutions were made daily from the stock solutions and incubated throughout an experiment in a water bath in the experimental tank. One ml of seawater was removed from one branch of solenoid tubing and replaced with 1 ml of amino acid stimulus to maintain constant volume. The solenoid valves were then triggered, which directed the water flow through the branch that contained the amino acid. The bolus of amino acid was transported through the odor delivery pipette, to the olfactory epithelium of the animal. Successive amino acid stimuli were administered two minutes after the EOG trace returned to approximately baseline level. Amino acid stimuli became diluted during transport from the injection site to the tip of the odor delivery pipette. To quantify the dilution factor, 1.0 ml of dye solution was injected into one of the solenoid branches in place of an amino acid stimulus and samples were collected from the odor delivery pipette at one-second intervals. The absorbance of the samples was measured with a spectrophotometer, and the dilution factor was calculated using the ratio of absorbance of the most concentrated sample solution to the stock dye solution. Stimuli delivered to the olfactory epithelium of the fish were diluted to 6% of their injected concentration. Therefore injecting a 10 M solution would present a 10 -4.2 M stimulus to the olfactory epithelium. 34 -3 The relative effectiveness of amino acid stimuli was tested by quantifying EOG responses -3 to each of the twenty amino acids at an injection concentration of 10 M. To determine the concentration-response relationships and olfactory threshold, five or six highly stimulatory amino -7 -3 acids were subsequently tested at increasing injection concentration from 10 M to 10 M for -3 each animal. A 10 M alanine standard was administered after approximately every fifth amino acid to determine the relative responsiveness of the tested amino acids throughout the experiment. The response to a 1.0 ml injection of seawater, the control, was also recorded periodically throughout the experiment. Analysis To compare the relative effectiveness of the twenty amino acid stimuli for each fish, the response magnitude of each test amino acid was expressed as a percent of the alanine standard (Silver, 1979). The response to alanine was recorded periodically throughout each experiment, as the magnitude of the response to the standard could change over experimental time. Thus, responses to amino acid test stimuli were taken as a percent of a calculated response to the alanine standard. This calculated response to the standard was obtained by determining the response magnitude at the exact time of the test stimulus using a regression of the alanine responses (response magnitude vs. time) preceding and following the test stimulus. Relative responses for each of the twenty amino acids were averaged for each species, and those averages were compared among amino acids within each species using a one-way ANOVA. Bonferroni t-tests were used for post hoc comparisons to account for the large number of pairwise comparisons among the twenty amino acids (Systat Software, Inc., San Jose, CA, USA) (Zar, 1999). Concentration-response relationships of five or six of the most stimulatory amino acids were determined for each species. Olfactory thresholds were calculated by regressing the amino acid concentration-response curve for each of the amino acids to its intersection with the control response to seawater for each individual animal. We employed Pearson product moment correlation tests to ascertain whether threshold to each amino acid varied with body size for each 35 species. Olfactory thresholds to alanine, phenylalanine, and serine were calculated for all five species and consequently were each compared among species using a one-way Kruskal-Wallis ANOVA on Ranks. Threshold data for the three amino acids were pooled both for each species and for each amino acid and were compared using one-way Kruskal-Wallis ANOVAs on Ranks. RESULTS Morphology Olfactory organs were dissected from five elasmobranch species (n ≥ 9), and the mean number of lamellae and total lamellar surface areas were quantified (Table 2.1). Lamellae from all five species were largest in the center of the organ and tapered in size towards the medial and lateral ends. Epithelial pigmentation differed among species: the three batoid species exhibited a predominantly white/tan epithelium whereas the epithelium of the two shark species was characterized by a black/brown pigmentation. All five species differed significantly from each other in the number of lamellae that comprise their olfactory organs (ANOVA, F 4,49= 694.258, P<0.001; Tukey test, P<0.001 for all comparisons). Raja eglanteria possessed the fewest lamellae (28.8 ± 0.63) and S. tiburo the most (68.6 ± 3.35). The two shark species had significantly more lamellae than the three batoid species (Mann-Whitney, U=140.00, P<0.001). Total lamellar surface area scaled positively as a power function with body size (DW or TL) for all species (Fig. 2.2). Total lamellar surface area differed significantly within the batoids (ANCOVA, F2,27=41.662, P<0.001); R. eglanteria possessed a significantly smaller mean total lamellar surface area than both U. jamaicensis and D. sabina (LSD test, P<0.001 for both comparisons). Within the sharks, N. brevirostris had a significantly smaller mean total lamellar surface area than S. tiburo (ANCOVA, F1,20=65.023, P<0.001). Electrophysiology: Relative effectiveness of amino acids EOG responses were recorded from the olfactory epithelium of five elasmobranch species (n≥6 per species) to twenty common amino acids. A typical EOG response is characterized by a rapid negative potential followed by a slower recovery back to baseline. The larger the response magnitude, the longer the trace took to rebound to baseline level. The 36 magnitude of the EOG response to the same stimulus was approximately three times greater in the two shark species compared to the three batoid species. For each of the five species, mean EOG response magnitudes to the twenty amino acids varied significantly (ANOVA, P<0.001 for all 5 spp.), with alanine and serine being particularly stimulatory. Pair-wise comparisons among amino acids within a species are illustrated in Fig. 2.3. Electrophysiology: Concentration-response relationships and olfactory threshold The concentration-response relationships for five or six highly stimulatory amino acids were very similar within each species (Fig. 2.4). The logarithm of the EOG response increased with a logarithmic increase in stimulus concentration. Olfactory thresholds to these amino acids were estimated for all five species (Fig. 2.5). The olfactory thresholds to amino acids did not vary with body size of teleost fishes (Hara, 1994) nor for any of our species (Pearson Product Moment Correlation, P>0.05 for all); therefore, body size was not used as a covariate during our analyses. Mean thresholds to the most stimulatory amino acids ranged between 10 -9.0 and 10 -6.9 M for the five species (Table 2.2). Olfactory thresholds to alanine, phenylalanine, and serine did not differ significantly among species (alanine: Kruskal-Wallis, H4 = 4.035, P =0.401; phenylalanine: Kruskal-Wallis, H4 = 1.909, P =0.753; serine: Kruskal-Wallis, H4 = 9.051, P =0.060). As a result, the thresholds for all five species were pooled for each of the three amino acids; the median thresholds to alanine (10 -7.8 ), phenylalanine (10 -7.9 ), and serine (10 -7.2 ) were significantly different (Kruskal-Wallis, H2 = 6.808, P =0.033); however the Dunn‟s Method post hoc test did not detect any significant differences in the pair-wise comparisons. Olfactory thresholds to the three amino acids were also pooled for each species, and again, there were no significant differences in olfactory threshold among species (Kruskal-Wallis, H4 = 6.365, P =0.174). DISCUSSION Olfaction plays an integral role for elasmobranchs in food localization (Parker, 1913; Sheldon, 1909; Sheldon, 1911) and also likely in mating (Johnson and Nelson, 1978; Kajiura et al., 2000), predator detection (Rasmussen and Schmidt, 1992), and in homing, and navigation (Edrén and Gruber, 2005). Olfaction is considered especially important as a distant sense since 37 chemical signals can become entrained in currents and transported much farther in the marine environment than mechanical or electrical signals (Hueter et al., 2004). This enables elasmobranchs to detect chemical cues emanating from distant sources in their expansive environment. Elasmobranchs are often reputed to possess greater olfactory sensitivity than teleost fishes, though sensitivity has been estimated in only five elasmobranch species (Nikonov et al., 1990; Silver, 1979; Tricas et al., 2009; Zeiske et al., 1986). Also, several recent morphological studies assumed that interspecific differences in the size of elasmobranch olfactory structures confer differences in olfactory sensitivity (Kajiura et al., 2005; Schluessel et al., 2008; Theiss et al., 2009). This study is the first to address these assumptions by comparing the olfactory morphology and physiology of five phylogenetically diverse elasmobranch species. We found that elasmobranchs and teleost fishes have comparable amino acid thresholds and that gross epithelial surface area (i.e. not taking into account secondary lamellae) is not a good predictor of olfactory sensitivity. This study compared the number of lamellae and lamellar surface area of five phylogenetically diverse elasmobranch species from similar near-shore habitats. Whereas lamellar surface area increases with body size of an elasmobranch, the number of lamellae does not; therefore body size was not included as a covariate during analyses (Fishelson and Baranes, 1997; Schluessel et al., 2010). Our results corroborate those of previous studies, which found interspecific differences in the number of lamellae and surface area for elasmobranchs (Kajiura et al., 2005; Schluessel et al., 2008; Theiss et al., 2009). A recent study (Schluessel et al., 2008) quantified the number of lamellae and epithelial surface area for 21 elasmobranch species and concluded that those factors did not correlate with phylogeny, but did with habitat (i.e., benthopelagic species possessed more lamellae and greater epithelial surface area than benthic species). When grouped by habitat, the two shark species in this study (bentho-pelagic) possessed significantly more lamellae than the three batoid species (benthic), supporting the results of Schluessel et al. (2008). 38 Teleost fishes also exhibit a wide diversity in the surface area and arrangement of the olfactory epithelium (Hansen and Zielinski, 2005; Kleerekoper, 1969; Yamamoto, 1982). These variations in teleost olfactory organ morphology were suggested to correlate with either ecology or phylogeny (Yamamoto, 1982; Zielinski and Hara, 2001). In their survey of olfactory morphology of over 70 species of teleosts, Hansen and Zielinski (2005) found that number of lamellae did not correlate with phylogeny or ecology in teleosts and they could not provide an alternative explanation for the wide variation in lamellar configuration. Studies on elasmobranch olfactory morphology have attempted to correlate differences in lamellar surface area with olfactory threshold (Kajiura et al., 2005; Schluessel et al., 2008; Theiss et al., 2009) even though the lack of correlation was previously demonstrated in teleost species (Hara, 1994). The rationale behind this proposed correlation is that a species with a greater lamellar surface area should possess a greater number of ORNs and molecular olfactory receptors, which would increase the probability of odorant binding, and thus the ability to detect odorants at a lower concentration than species with less lamellar surface area. Although previous studies found interspecific differences in gross lamellar surface area (Kajiura et al., 2005; Schluessel et al., 2008; Theiss et al., 2009), the number and density of ORNs, extent to which secondary lamellar folding increased surface area, and olfactory thresholds were not quantified. In this study, we tested the hypothesis that lamellar surface area positively correlates with olfactory sensitivity. We found that although interspecific variation in olfactory lamellar surface area occurred, olfactory thresholds to amino acid odorants did not differ significantly among the five species, with all species demonstrating similar thresholds of between 10 -6.9 10 -9.0 and M (Table 2.2). Even though olfactory organs of elasmobranchs are characterized by secondary lamellar folding that greatly increases the actual surface area of the lamellae, these organs are not more sensitive to amino acid stimuli than the smaller organs of teleost fishes that do not possess secondary lamellae (Hansen and Zielinski, 2005; Hara, 1994; Yamamoto, 1982). Also, olfactory threshold did not correlate significantly with body size for any species; individuals 39 within a species were all selected to be of a similar size, which may obscure any potential size effects. Greater lamellar surface area may not confer greater olfactory sensitivity because it does not necessarily translate to a greater number or density of ORNs; also ORN quantity is only one of several factors that affect sensitivity. First, the background level of odorants in the environment was shown to affect the olfactory threshold of fishes. An increase in the background level of amino acids would cause the animal‟s olfactory receptors to become adapted to that amino acid concentration. This would cause an increase in the amino acid olfactory threshold to the concentration of the adapting stimulus (Caprio, 1982). Second, sensitivity is also determined by the number of ORNs converging onto a specific glomerulus in the olfactory bulb, where the axons of ORNs synapse with the dendrites of mitral/tufted neurons (Hamdani and Doving, 2007). With a high convergence ratio of ORNs, glomeruli in the bulb would have a greater chance of being stimulated at low odor concentrations. Third, olfactory receptors also possess various binding affinities for different odorants, as is the case with fish olfactory receptors and amino acids (Bruch and Rulli, 1988; Cagan and Zeiger, 1978). If a given receptor has a high binding affinity for a particular odorant and occurs in sufficient numbers in the olfactory epithelium, the animal would experience a high sensitivity to that odorant since a relatively low concentration of the odorant is likely adequate to maximally occupy the receptor binding site and trigger a physiological response. Finally, we only determined the olfactory responses of elasmobranchs to amino acids; it is possible that their olfactory thresholds to other types of odorants may be different. The concentration-response relationships for the five or six highly stimulatory amino acids tested were very similar within each species (Fig. 2.4). There appears to be differences in relative effectiveness at lower concentrations compared to that at 10 -4.2 M (Fig. 2.3); however, due to the similarity in relative effectiveness for these amino acids, these differences were not significant. The responses of each species to the five or six highly stimulatory amino acids tested at multiple concentrations increased predictably with increase in stimulus concentration. Some 40 interspecific variability in the relative effectiveness of the twenty amino acids tested at 10 -4.2 M was observed, but the neutral amino acids, such as alanine, serine, and methionine, were generally highly stimulatory (Table 2.3). Neutral amino acids were previously demonstrated to be especially potent stimuli for a few elasmobranch species and for teleost fishes (Caprio and Byrd, 1984; Silver, 1979; Tricas et al., 2009; Zeiske et al., 1986). Tricas et al. (2009) found that the response of scalloped hammerhead sharks to cysteine was about twice that for alanine; however, cysteine was a less effective stimulus for the five species we tested (44 - 75% of alanine), which included another sphyrnid species. Valine, proline, and isoleucine were some of the least effective stimuli for the species we tested and for scalloped hammerhead sharks and teleosts as well. All three are neutral, nonpolar, hydrophobic amino acids, which are characteristics shared by alanine, a highly stimulatory amino acid; however, valine and isoleucine have branched sidechains in contrast to alanine‟s short side chain. Also, proline is considered an imino acid due to the presence of a secondary amine group. Although the molecular characteristics of the amino acids may not be good predictors of relative effectiveness as olfactory stimuli, our results on the relative effectiveness of amino acids support those of the previous elasmobranch olfactory physiology studies (Silver, 1979; Tricas et al., 2009; Zeiske et al., 1986). -9 -7 Amino acid thresholds for over thirty species of teleosts range from ~10 to 10 M (Hara, 1994). This amino acid concentration range approximates the level of free amino acids in seawater (Hara, 1994; Kuznetsova et al., 2004; Pocklington, 1971). Since the olfactory threshold of a fish varies depending on the background level of odorants (Caprio, 1982), and many fishes are subject to similar environmental amino acid levels, both teleosts and elasmobranchs converged on similar amino acid sensitivities. As a result, we cannot support the claim that elasmobranchs demonstrate superior olfactory sensitivities compared to teleost fishes. This finding is contrary to assumptions that persist in the scientific literature, popular science media, and culturally. Elasmobranchs are thought to have a particularly acute sense of smell, and while -9 their ability to detect odors at 10 M is remarkable, they are no more sensitive than teleosts. 41 This study examined the olfactory morphology and physiology of five elasmobranch species. We found that elasmobranchs, like teleost fishes, exhibit interspecific differences in the olfactory organs; however these differences did not correlate with differences in amino acid threshold. Although elasmobranchs are reputed to possess greater olfactory sensitivities than bony fishes, they demonstrate comparable amino acid thresholds as teleosts, further highlighting the olfactory system parallels between these two groups. Future studies should test the responses of elasmobranchs to other biologically relevant odorants, such as bile salts, and use cross-adaptation techniques to determine whether elasmobranchs possess similar amino acid receptors as teleost fishes. 42 REFERENCES Bell, M. A. (1993). Convergent evolution of nasal structure in sedentary elasmobranchs. Copeia 1, 144-158. Bronshtein, A. A. (1976). Some peculiarities of the fine structure of olfactory organ in elasmobranchs. ZH Evol Biokhim Fiz 12, 63-67. Bruch, R. C. and Rulli, R. D. (1988). Ligand-Binding Specificity of a Neutral L-Amino-Acid Olfactory Receptor. Comp. Biochem. Physiol. B-Biochem. Mol. Biol. 91, 535-540. Cagan, R. H. and Zeiger, W. N. (1978). Biochemical Studies of Olfaction - Binding Specificity of Radioactively Labeled Stimuli to an Isolated Olfactory Preparation from Rainbow-Trout (Salmo-Gairdneri). Proc. Natl. Acad. Sci. U. S. A. 75, 4679-4683. Caprio, J. (1982). High sensitivity and specificity of olfactory and gustatory receptors of catfish to amino acids. In Chemoreception in Fishes, (ed. T. J. Hara), pp. 109-134. Amsterdam: Elsevier. Caprio, J. (1995). In vivo olfactory and taste recordings in fish. In Experimental cell biology of taste and olfaction, eds. A. I. Spielman and J. G. Brand), pp. 251-261. Boca Raton: CRC Press. Caprio, J. and Byrd, R. P. (1984). Electrophysiological Evidence for Acidic, Basic, and Neutral Amino-Acid Olfactory Receptor-Sites in the Catfish. J. Gen. Physiol. 84, 403-422. Compagno, L. J. V. (2002). Sharks. In The Living Marine Resources of the Western Central Atlantic, vol. 1: Introduction, Molluscs, Crustaceans, Hagfishes, Sharks, Batoid Fishes and Chimaeras (ed. K. E. Carpenter), pp. 357-505. Rome, Italy: FAO. Edrén, S. M. C. and Gruber, S. H. (2005). Homing ability of young lemon sharks, Negaprion brevirostris. Environ. Biol. Fishes 72, 267-281. Fishelson, L. and Baranes, A. (1997). Ontogenesis and cytomorphology of the nasal olfactory organs in the Oman shark, Iago omanensis (Triakidae), in the Gulf of Aqaba, Red Sea. Anat. Rec. 249, 409-421. 43 Hamdani, E. H. and Doving, K. B. (2007). The functional organization of the fish olfactory system. Prog. Neurobiol. 82, 80-86. Hansen, A. and Zielinski, B. (2005). Diversity in the olfactory epithelium of bony fishes: Development, lamellar arrangement, sensory neuron cell types and transduction components. J. Neurocytol. 34, 183-208. Hara, T. J. (1994). The Diversity of Chemical Stimulation in Fish Olfaction and Gustation. Rev. Fish Biol. Fisher. 4, 1-35. Hodgson, E. S. and Mathewson, R. F. (1978). Electrophysiological studies of chemoreception in elasmobranchs. In Sensory biology of sharks, skates and rays, eds. E. S. Hodgson and R. F. Mathewson), pp. 227-266. Washington D.C.: Government Printing Office. Hueter, R. E., Mann, D. A., Maruska, K. P., Sisneros, J. A. and Demski, L. S. (2004). Sensory Biology of Elasmobranchs. In Biology of Sharks and Their Relatives, eds. J. C. Carrier J. A. Musick and M. R. Heithaus). Boca Raton, London, New York, Washington D.C.: CRC Press. Johnson, R. H. and Nelson, D. R. (1978). Copulation and possible olfaction-mediated pair formation in two species of carcharhinid sharks. Copeia 3, 539-542. Kajiura, S. M., Forni, J. B. and Summers, A. P. (2005). Olfactory morphology of carcharhinid and sphyrnid sharks: Does the cephalofoil confer a sensory advantage? J. Morphol. 264, 253263. Kajiura, S. M., Sebastian, A. P. and Tricas, T. C. (2000). Dermal Bite Wounds as Indicators of Reproductive Seasonality and Behaviour in the Atlantic Stingray, Dasyatis sabina. Environ. Biol. Fishes 58, 23-31. Kleerekoper, H. (1969). Olfaction in fishes. Bloomington: Indiana University Press. Kuznetsova, M., Lee, C., Aller, J. and Frew, N. (2004). Enrichment of Amino Acids in the Sea Surface Microlayer at Coastal and Open Ocean Sites in the North Atlantic Ocean. Limnol Oceanogr 49, 1605-1619. 44 Lisney, T. J., Bennett, M. B. and Collin, S. P. (2007). Volumetric shift of sensory brain areas indicates ontogenetic shifts in the relative importance of sensory systems in elasmobranchs. The Raffles Bulletin of Zoology Supplement No. 14, 7-15. Lisney, T. J. and Collin, S. P. (2006). Brain morphology in large pelagic fishes: a comparison between sharks and teleosts. J Fish Biol 68, 532-554. McEachran, J. D. and de Carvalho, M. R. (2002). Batoid Fishes. In The Living Marine Resources of the Western Central Atlantic, vol. 1: Introduction, Molluscs, Crustaceans, Hagfishes, Sharks, Batoid Fishes and Chimaeras (ed. K. E. Carpenter), pp. 507–589. Rome, Italy: FAO. Meng, Q. and Yin, M. (1981). A study of the olfactory organ of the shark. Transactions of the Chinese Ichthyological Society 2, 1-24. Nikonov, A. A., Illyin, Y. N., Zherelove, O. M. and Fesenko, E. E. (1990). Odour thresholds of the black sea skate (Raja clavata). Electrophysiological study. Comp. Biochem. Physiol. 95A, 325-328. Northcutt, R. G. (1977). Elasmobranch central nervous system organization and its possible evolutionary significance. Am. Zool. 17, 411-429. Northcutt, R. G. (1978). Brain organization in the cartilagenous fishes. In Sensory biology of shark, skates, and rays, eds. E. S. Hodgson and R. F. Mathewson), pp. 117-193. Washington, D. C.: Government Printing Press. Parker, G. H. (1913). The directive influence of the sense of smell in the dogfish. In Bulletin of the United States Bureau of Fisheries, vol. 33 (ed. H. M. Smith, Commissioner), pp. 63-68. Washington, D. C.: Government Printing Office. Pocklington, R. (1971). Physical Sciences: Free Amino-acids dissolved in North Atlantic Ocean Waters. 230, 374-375. Rasmussen, L. E. L. and Schmidt, M. J. (1992). Are sharks chemically aware of crocodiles? In Chemical Signals in Vertebrates VI, vol. VI eds. R. L. Doty and D. Muller-Schwarze), pp. 335-341. New York: Plenum Press. 45 Schluessel, V., Bennett, M. B., Bleckmann, H., Blomberg, S. and Collin, S. P. (2008). Morphometric and ultrastructural comparison of the olfactory system in elasmobranchs: The significance of structure-function relationships based on phylogeny and ecology. J. Morphol. 269, 1365-1386. Schluessel, V., Bennett, M. B., Bleckmann, H. and Collin, S. P. (2010). The role of olfaction throughout juvenile development: Functional adaptations in elasmobranchs. J. Morphol. 271, 451-461. Sheldon, R. E. (1909). The reactions of the dogfish to chemical stimuli. J Comp. Neurol. Psycho. 19, 273-311. Sheldon, R. E. (1911). The sense of smell in Selachians. J. Exp. Zool. 10, 51-62. Silver, W. L. (1979). Olfactory Responses from a Marine Elasmobranch, the Atlantic Stingray, Dasyatis sabina. Mar. Behav. Physiol. 6, 297-305. Silver, W. L., Caprio, J., Blackwell, J. F. and Tucker, D. (1976). Underwater Electro-Olfactogram Tool for Study of Sense of Smell of Marine Fishes. Experientia 32, 1216-1217. Tester, A. L. (1963). Olfaction, gustation and the common chemical sense in sharks. In Sharks and Survival, (ed. P. W. Gilbert), pp. 243-254. Boston, MA: DC Health. Theisen, B., Zeiske, E. and Breucker, H. (1986). Functional morphology of the olfactory organs in the spiny dogfish (Squalus acanthius L.) and the small-spotted catshark (Scyliorhinus canicula (L.)). Acta Zool. 67, 73-86. Theiss, S. M., Hart, N. S. and Collin, S. P. (2009). Morphological Indicators of Olfactory Capability in Wobbegong Sharks (Orectolobidae, Elasmobranchii). Brain Behav. Evolut. 73, 91-101. Tricas, T. C., Kajiura, S. M. and Summers, A. P. (2009). Response of the hammerhead shark olfactory epithelium to amino acid stimuli. J. Comp. Physiol. A 195, 947-954. Yamamoto, M. (1982). Comparative morphology of the peripheral olfactory organ in teleosts. In Chemoreception in fishes, (ed. T. J. Hara), pp. 39-59. New York, NY: Elsevier. 46 Yopak, K. E., Lisney, T. J., Collin, S. P. and Montgomery, J. C. (2007). Variation in brain organization and cerebellar foliation in chondrichthyans: Sharks and holocephalans. Brain Behav. Evolut. 69, 280-300. Zar, J. H. (1999). Biostatistical analysis. Upper Saddle River, New Jersey: Prentice Hall. Zeiske, E., Caprio, J. and Gruber, S. H. (1986). Morphological and electrophysiological studies on the olfactory organ of the lemon shark, Negaprion brevirostris. In Indo-Pacific Fish Biology: Proceedings of the Second International Conference on Indo-Pacific Fishes, eds. T. Uyeno R. Arai T. Taniuchi and K. Matsuura), pp. 381-391. Tokyo: Ichtyological Society of Japan. Zielinski, B. and Hara, T. J. (2001). The Neurobiology of Fish Olfaction. In Sensory Biology of Jawed Fishes: New Insights, eds. B. G. Kapoor and T. J. Hara), pp. 347-366: Science Publishers, Inc. 47 Table 2.1 The number and appearance of the olfactory lamellae for the species used in this study. 48 x Table 2.2 Mean olfactory thresholds (10 M) of five elasmobranch species to highly stimulatory amino acids after compensation for stimulus dilution. 49 Table 2.3 Ranked mean EOG responses of five elasmobranch species to 20 amino acids. Note: Each amino acid has been ranked from most stimulatory (1) to least stimulatory (20) for each species. Highlighted bars indicate amino acids which were consistently most (yellow) or least (gray) stimulatory for all five species. 50 Figure 2.1 Experimental apparatus used to record the electro-olfactogram (EOG). Animals were secured onto a platform in an experimental seawater (SW) tank and ventilated with SW. The tank was supplied with mechanically and chemically filtered SW through a PVC manifold. One manifold arm delivered a constant flow of SW through a flow meter; paired computer-controlled, three-way solenoid valves; and an odor delivery pipette (ODP, inset), which was inserted into the incurrent naris. Amino acid stimuli were delivered through the ODP to the olfactory organ. Paired, non-polarizable, Ag-AgCl electrodes recorded the EOG. The glass tip of the active electrode (Act E, inset) was positioned in the SW immediately above the olfactory epithelium while the glass tip of the reference electrode (Ref E, inset) contacted the skin adjacent to the naris. The output was differentially amplified (1000-10000x), filtered (high pass 0.1 Hz, low pass 0.1 kHz, 50/60 Hz), digitized (1 kHz) and recorded. 51 Figure 2.2 Total lamellar surface area for the five elasmobranch species tested scaled positively as a power function with body size (disc width or total length). Regression lines are indicated for 1.8988 2.4689 1.5725 R. eglanteria: y = 0.0402x ; U. jamaicensis: y = 0.0297x ; D. sabina: y = 0.242x ; N. 1.4371 1.3342 brevirostris: y = 0.0947x ; S. tiburo: y = 0.3052x . Outliers were excluded from the regressions for R. eglanteria, D. sabina, and N. brevirostris. Raja eglanteria had a significantly smaller mean total lamellar surface area than U. jamiacensis and D. sabina. Negaprion brevirostris had a significantly smaller mean total lamellar surface area than S. tiburo. 52 Figure 2.3 Mean EOG responses (+ s.d.) for five elasmobranch species (n≥6) to 20 amino acids and the SW control. Response magnitudes are expressed as a percent of the response to the -3 standard (10 M alanine). Colors represent the results of pair-wise comparisons using Bonferroni t-tests. For each species, amino acids that share a bar color do not differ significantly from each other. ala alanine, arg arginine, asn asparagine, asp aspartic acid, cys cysteine, gln glutamine, glu glutamic acid, gly glycine, his histidine, ile isoleucine, leu leucine, lys lysine, met methionine, phe phenylalanine, pro proline, ser serine, thr threonine, trp tryptophan, tyr tyrosine, val valine, sw seawater. Line drawings of batoids are modified from McEachran and de Carvalho (2002), and line drawings of sharks are modified from Compagno (2002). 53 Figure 2.4 Concentration-response relationships of five or six highly stimulatory amino acids for each species. Response magnitudes represent the mean response for each amino acid at each -3 concentration and are expressed as a percent of the standard (10 M alanine). Refer to Fig. 3 for statistical differences. 54 Figure 2.5 Representative EOG concentration-response curve of a S. tiburo individual expressed -3 as a percent of the standard (10 M alanine). The magnitude of the log EOG response is linearly related to the log amino acid stimulus concentration. The horizontal dashed black line indicates the averaged response to the seawater control. The olfactory threshold is calculated as the point where the regression line for the best fit line of the response intersects the averaged response to the seawater (SW) control. The inset shows representative EOG responses to the SW control and to increasing log concentrations of L-alanine. Based on absorbance calculations of diluted dye, all stimuli were diluted to 6% of their injected concentration at the entrance to the incurrent naris. Plotted are the estimated diluted stimulus concentrations at arrival to the olfactory organ. 55 CHAPTER 3 SENSITIVITY AND SPECIFICITY OF THE ELASMOBRANCH OLFACTORY EPITHELIUM TO BILE SALTS ABSTRACT Odor detection in vertebrates occurs when odorants enter the nose and bind to molecular olfactory receptors (ORs) on the cilia or microvilli of olfactory receptor neurons (ORNs). Several vertebrate groups possess multiple, morphologically distinct types of ORNs. In teleost fishes, these different ORN types detect specific classes of biologically relevant odorants, such as amino acids, nucleotides and bile salts. For example, bile salts are reported to be detected exclusively by ciliated ORNs. The olfactory epithelium of elasmobranch fishes (sharks, rays, and skates) is comprised of microvillous and crypt ORNs, but lacks ciliated ORNs; thus, it was questioned whether the olfactory system of this group of fishes is capable of detecting bile salts. The present investigation clearly indicates that the olfactory system of a representative shark and stingray species do detect and respond to bile salts. Additionally, these species detect glycine-conjugated, taurine-conjugated, and non-conjugated bile salts as do teleosts. These elasmobranchs are less sensitive to the tested bile salts than reported for either agnathans or teleosts, but this may be due to the particular bile salts selected in this study, as elasmobranch-produced bile salts are commercially unavailable. Cross-adaptation experiments indicate further that the response to bile salts are independent of those to amino acids, a major class of odorant molecules for all tested fishes. INTRODUCTION The olfactory system functions similarly for most vertebrates and mediates several important life history behaviors, such as feeding, reproduction, and predator avoidance (Ache and 56 Young, 2005). Chemicals, either volatized in air for tetrapods or dissolved in water for fishes and aquatic amphibians, enter the nose and bind to molecular G-protein coupled olfactory receptors (ORs) on the cilia or microvilli of olfactory receptor neurons (ORNs) in the olfactory epithelium (OE) (Eisthen, 2002). Vertebrate groups, including lampreys, teleost fishes, lungfish, frogs, and mammals, possess two morphologically different types of ORNs coupled to specific G-protein α subunits: ciliated ORNs utilizing the Gαolf transduction cascade, and microvillous ORNs coupled to either Gαo, Gαi, or Gαq (Eisthen, 2004). Lampreys and actinopterygian (but not sarcoptergygian) fishes also possess a third type of ORN, the crypt cell, which use the Gαo or Gαq transduction cascades (Ferrando et al., 2006; Hansen et al., 2004; Hansen and Finger, 2000; Laframboise et al., 2007; Zeiske et al., 2003). The morphologically different types of ORNs are thought to mediate responses to specific odorant classes. The olfactory system of fishes is sensitive to several types of odorants, including amino acids, polyamines, bile salts, prostaglandins, steroids and nucleotides (Hara, 1994; Rolen et al., 2003; Zielinski and Hara, 2006). Amino acids, a feeding stimulant in fishes (Zielinski and Hara, 2006), are detected primarily by microvillous ORNs (Lipschitz and Michel, 2002; Sato and Suzuki, 2001), but also possibly by ciliated and crypt ORNs (Hansen et al., 2004; Vielma et al., 2008). In contrast, several studies demonstrated that for teleosts, bile salts are detected only by ciliated ORNs (Døving et al., 2011; Hansen et al., 2003; Sato and Suzuki, 2001). Additionally, cross-adaptation and mixture experiments confirm that teleosts possess ORs for bile salts that are independent from those for amino acids, prostaglandins, gonadal steroids, and polyamines (Laberge and Hara, 2004; Michel and Derbidge, 1997; Zhang and Hara, 2009). Bile salts, which are produced throughout the vertebrate clade, are biliary steroids created in the liver by the oxidation of cholesterol to facilitate intestinal absorption of lipids and fat-soluble vitamins (Hagey et al., 2010; Haslewood, 1967; Hofmann et al., 2010). Bile salts are typically reabsorbed and reused by the enterohepatic system, but teleost fishes excrete a portion of their bile salts in their urine or feces (Velez et al., 2009; Zhang et al., 2001). These excreted bile salts are potent olfactory stimuli, as several teleost species have demonstrated a high 57 olfactory specificity and sensitivity to these compounds in the nanomolar range and lower (Huertas et al., 2010; Zhang and Hara, 2009). Similarly, sea lampreys, whose OE contains the same three ORN types as teleosts (Laframboise et al., 2007), are extremely sensitive to bile salts (threshold: 10 -13 -1 mol l ), employing them as pheromones to attract mates and guide adults to spawning streams (Li et al., 2002; Siefkes and Li, 2004; Sorensen et al., 2005). Teleosts may also use bile salts as pheromones (Hara, 1994; Huertas et al., 2007; Sorensen and Caprio, 1997; Sorensen and Stacey, 2004; Zhang et al., 2001). For example, bile salts released by salmonids were suggested to mediate the homeward migration of conspecifics to their natal stream (Døving et al., 1980). Elasmobranch fishes (sharks, rays and skates) possess an olfactory system that is morphologically and physiologically similar to that of teleosts (Hansen et al., 2005; Meredith and Kajiura, 2010; Schluessel et al., 2008; Silver, 1979; Takami et al., 1994; Tricas et al., 2009; Zeiske et al., 1986; Zeiske et al., 1987; Zielinski and Hara, 2006). Like other fishes, elasmobranchs possess microvillous ORNs that are likely coupled to Gα o proteins and crypt ORNs; however, they are unique in their lack of ciliated ORNs and the corresponding Gαolf expression present in the olfactory epithelium of most vertebrate taxa (Ferrando et al., 2006; Ferrando et al., 2009; Schluessel et al., 2008). The OE of elasmobranchs is highly sensitive to amino acid odorants (Meredith and Kajiura, 2010; Silver, 1979; Tricas et al., 2009; Zeiske et al., 1986), which is likely due to the presence of microvillous ORNs. Since elasmobranchs lack ciliated ORNs and the associated expression of Gαolf , it is unknown whether they are able to detect bile salts, though the biological relevance of this odorant class for agnathans and teleosts makes it seem likely. This study tested the hypotheses that the olfactory systems of elasmobranch fishes are able to detect bile salt odorants and that the receptor mechanism for bile salts is independent from that for amino acids. 58 MATERIALS AND METHODS Animal Collection We tested bile salts on the olfactory systems of two distantly related elasmobranch species, the bonnethead shark, Sphyrna tiburo (Linnaeus 1758) and the Atlantic stingray, Dasyatis sabina (Lesueur 1824). Both species were collected from south Florida near shore waters using gill-netting and seining techniques. All animals were transported to the Florida Atlantic University Marine Lab at the Gumbo Limbo Environmental Complex (Boca Raton, FL, USA), maintained in tanks with flow-through seawater, and fed a diet of shrimp and squid daily to satiation. All experiments were conducted in accordance with an approved IACUC protocol from Florida Atlantic University (A08-05). Electro-olfactogram The underwater electro-olfactogram (EOG) technique was employed to record the olfactory responses of the two species to bile salt odorants (Fig.3.1). Prior to experimentation, an animal was injected (intramuscularly or intravenously) with the paralytic, pancuronium bromide -1 (0.03 mg kg ). Immediately upon cessation of active ventilation, the animal was transferred to the experimental tank and secured ventral side up to a submerged platform. The acrylic experimental tank (89 x 43 x 21 cm) was supplied with flow-through seawater which was mechanically (25 m polyscreen) and chemically (activated charcoal) filtered. The animal was electrically grounded via a silver wire in the tank. Seawater was delivered to the tank through two arms of a PVC manifold with the flow for each arm controlled by a ball valve. One arm provided ventilatory water flow via the mouth (S. tiburo) or spiracles (D. sabina) and over the gills, and a second manifold arm provided seawater flow through the tank which was continuously drained to reduce chemical accumulation. Either seawater or an adapting amino acid was delivered from one of two large buckets through a flow meter to an odor delivery pipette that provided a constant background flow over the olfactory organ. The odor delivery pipette, mounted in a micromanipulator, was positioned with the tip in the incurrent naris, and the water flow was -1 regulated to 2 ml s (Tricas et al., 2009). 59 The active EOG electrode, a non-polarizable Ag-AgCl electrode (E45P-M15NH, Warner Instruments, Hamden, CT, USA) fitted with a seawater/agar-filled glass capillary tube, was positioned in the excurrent naris in the water just above the olfactory epithelium and recorded the animal‟s responses to odor stimuli. A similar reference electrode was positioned nearby in contact with the skin. The output from the two electrodes was differentially amplified 1000x (DP304, Warner Instruments), filtered (0.1 Hz - 0.1 kHz, 50/60 Hz) (DP-304, Warner Instruments & Hum Bug, Quest Scientific, North Vancouver, BC, Canada), digitized at 1 kHz (Power Lab® 16/30 model ML 880, AD Instruments, Colorado Springs, CO, USA) and recorded (Chart™ Software, AD Instruments). EOG responses were recorded from each individual to four commercially available bile salts that are known to be highly stimulatory to the teleost olfactory system (Rolen and Caprio, 2007; Zhang and Hara, 2009). The four bile salts represent three conjugation types: cholic acid (CA), a nonconjugated bile salt; glycochenodeoxycholic acid (GCDC), a glycine-conjugated bile salt; and taurochenodeoxycholic acid (TCDC) and taurolithocholic acid 3-sulfate (TLCS), both taurine-conjugated bile salts (Fig. 3.2A). The bile salts were applied individually over a background flow of either seawater or an adapting L-amino acid solution. Four L-amino acids, representing four side-chain structural variations, were selected as adapting stimuli: alanine (neutral), glutamic acid (acidic), arginine (basic), and phenylalanine (aromatic) (pH ~7.6). All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) and were ≥ 98% pure. Stock solutions of amino acids and bile salts were prepared with filtered seawater daily. For CA and TLCS, 0.1 mL of 100% methanol was the initial solvent. Test solutions were created by diluting the stock solutions to the experimental concentrations with filtered seawater (pH ~7.0). One ml of bile salt solution was injected into the tubing immediately above the odor delivery pipette and transported via the background flow to the olfactory epithelium, during which time it became further diluted. To quantify the dilution factor of the injected stimuli, 1.0 ml of fast green dye solution was injected into the tubing in place of a test stimulus and samples were collected every two seconds at the tip of the odor delivery pipette. The spectrophotometric 60 absorbance of the most concentrated sample was measured and its concentration was calculated to determine the dilution factor. Stimuli delivered to the olfactory epithelium of the fish were -4 -1 diluted to approximately 9% of their injected concentration. Therefore injecting a 10 mol l -5 -1 solution would present a ~10 mol l stimulus to the olfactory epithelium. Relative effectiveness The relative effectiveness of bile salt stimuli was tested by quantifying the EOG -4 -1 responses to each of four bile salt solutions at an injection concentration of 10 mol l . A 10 -3 -1 mol l alanine standard was administered throughout the experiment after approximately every fifth bile salt stimulus. To compare the relative effectiveness of the four bile salt stimuli for each fish, the response magnitude to each bile salt was expressed as a percentage of the alanine standard. The mean relative responses for each of the four bile salts were log transformed when necessary to achieve normality and compared within each species using a two-way analysis of variance (ANOVA). Holm-Sidak tests were used for post hoc comparisons (Systat Software, Inc., San Jose, CA, USA). The response to seawater and 0.1% methanol dissolved in seawater were also recorded periodically throughout the experiment to serve as control stimuli. Concentration-response relationships Concentration-response relationships were determined for each species by recording -6 -1 their EOG responses to the four bile salts at increasing injection concentrations from 10 mol l to -3 -1 10 mol l . Olfactory thresholds were calculated for each individual animal by regressing each bile salt concentration-response curve to its intersection with the control response to seawater. Olfactory thresholds were log transformed when necessary to achieve normality and compared within each species using a two-way ANOVA. Threshold data were then pooled for each species and compared using a Mann-Whitney Rank Sum Test (Systat Software, Inc., San Jose, CA, USA). Cross-adaptation Cross-adaptation experiments were employed to determine whether bile salt and amino acid odorants bind to independent or overlapping molecular olfactory receptors. The 61 experimental procedures for the cross-adaptation experiments paralleled those that were previously described for teleosts (Caprio and Byrd, 1984; Zhang and Hara, 2009). The concentrations of the bile salts were chosen as the nearest whole log concentration that elicited -3 -1 similar EOG response magnitudes as 10 mol l CA in order to reduce response variation due to differences in stimulatory effectiveness (Caprio and Byrd, 1984). After establishing the appropriate bile salt concentrations, EOG responses were recorded to the four bile salts administered at approximately three-minute intervals in a background flow of seawater (Fig. 3.2B.). The background flow was then switched to one of four adapting L-amino acids, each representing a side-chain structural group. The adapting amino acid was presented continuously over the olfactory epithelium for a minimum of 10 minutes resulting in a tonic EOG response during which phasic responses to the bile salts were recorded (Fig. 3.2C.). Once responses to all of the bile salts were recorded during a particular adapting regime, the background flow was returned to seawater for a minimum of 30 minutes to allow the olfactory receptors to de-adapt, or recover. The EOG responses to the bile salts delivered in a background flow of seawater were once again recorded to confirm that the animal was still responding at the pre-adaptation level. This procedure was repeated for each of the three remaining adapting regimes. To determine the magnitude of depression in the bile salt responses during amino acid adaptation, the EOG responses to the bile salts during adaptation were taken as a percentage of the responses during the unadapted state, after subtracting the control responses (Caprio and Byrd, 1984): Percent Unadapted Response (PUR) (adapted response control A) (unadapted response SW) 100 Control A represents the magnitude of the EOG response to an amino acid during adaptation with that same amino acid. SW is the response to an injection of seawater during the unadapted state (background flow of seawater). 62 Due to the low sample size of responses for each bile salt in each adapting regime (n≥2 each), data were pooled for each adapting regime and the responses to the bile salts in the unadapted state were compared to those during the adapted state using paired t-tests (Systat Software, Inc., San Jose, CA, USA). RESULTS Relative effectiveness EOG responses to four bile salts were recorded from the olfactory epithelium of D. sabina -4 -1 (n≥5) and S. tiburo (n=4). The mean EOG response magnitudes to the four 10 mol l bile salts varied significantly for both species (two-way ANOVA, P<0.001 for both species) and ranged from -3 -1 7-18% of the response to the 10 mol l alanine standard (Fig. 3.3). The responses of D. sabina to the bile salts were consistently greater than those of S. tiburo. TLCS (18% of Ala) and GCDC (13% of Ala) were most stimulatory to D. sabina and S. tiburo, respectively. Concentration-response relationships EOG responses of the two elasmobranch species to the four bile salts were tested at -6 -1 -3 -1 increasing injection concentration from 10 mol l to 10 mol l . The general shapes of the concentration-response relationships for the four bile salts were similar within each species (Fig. 3.4). As expected, the EOG response increased with increasing bile salt concentration. Cholic acids was the least effective bile salt for D. sabina compared to the other bile salts tested over multiple log steps of concentration. Mean olfactory thresholds to the bile salts estimated for both species ranged between 10 -7.2 -5.5 and 10 -1 mol l (n≥3) (Table 3.1). Olfactory thresholds to the four bile salts did not differ significantly within each species (two-way ANOVA, P=0.873 for D. sabina, P=0.070 for S. tiburo); therefore, the mean olfactory thresholds were pooled for each species for interspecific comparison. The pooled group of olfactory thresholds were significantly lower for D. sabina than those for S. tiburo (Mann-Whitney, U=190.00, P=0.020). Cross-adaptation The cross-adaptation protocol was employed to test whether bile salt and amino acid odorants interact with independent or overlapping molecular olfactory receptors. The mean EOG 63 responses to the four bile salts tested during adaptation with each of four L-amino acids were 89.7±7.1% for D. sabina and 102.5±6.5% for S. tiburo (Fig. 3.5; n≥2 for each bile salt in each adapting regime). For both species, the responses to the bile salts during the adapted state did not differ significantly from those during the unadapted state (P >0.05 for all tests) indicating that bile salts bind to molecular olfactory receptors different from those that bind to amino acids. DISCUSSION Elasmobranchs possess microvillous and crypt ORNs in their OE, but they lack the ciliated ORNs found in the OE of teleosts and most other vertebrates (Eisthen, 2004; Schluessel et al., 2008). Teleosts use ciliated ORNs to detect bile salt odorants, which serve as important olfactory cues (Døving et al., 1980; Hansen et al., 2003; Hansen and Zielinski, 2005; Sato and Suzuki, 2001). This study investigated whether elasmobranchs that lack ciliated ORNs are able to detect bile salt odorants. The two elasmobranch species tested responded to bile salts, despite lacking ciliated ORNs, though they were less sensitive to the particular bile salts used in this study than previously documented for teleosts and agnathans. In addition, elasmobranchs, like teleosts, possess molecular ORs sensitive to bile salts that are relatively independent of those that detect amino acids, which are food-related odorants; this demonstrated that even though they employ only two ORN morphotypes in their OE, neither of which are ciliated ORNs, elasmobranchs are still able to distinguish bile salts from other odorants with a high degree of specificity. All vertebrate classes produce bile salts which differ structurally from clade to clade. Within fishes, an evolutionary progression occurs from 5α bile alcohols produced by Agnathans to 5β bile alcohols of Chondrichthyans and C24 bile acids of Actinopterygians (Hagey et al., 2010). Specifically, sea lampreys produce C24 and C27 bile alcohols and hagfish produce 5α-myxinol disulfate, a C27 5α bile alcohol disulfate. Elasmobranch fishes primarily produce a C 27 bile alcohol, 5β-scymnol 27-sulfate (Bridgwater et al., 1962; Hagey et al., 2010; Hofmann et al., 2010). The most common bile salts produced by ray-finned fishes are C24 bile acids, such as cholic acid (used in this study) (Hagey et al., 2010; Haslewood, 1967). Teleosts also produce a 64 variety of other C24 bile salts, including taurine and glycine conjugates, and possess a high olfactory sensitivity to all of these compounds (Denton et al., 1974; Michel and Lubomudrov, 1995; Rolen and Caprio, 2007; Zhang and Hara, 2009; Zhang et al., 2001). Since teleost olfactory responses to bile salts are mediated by ciliated ORNs, which elasmobranchs lack, elasmobranchs must use either microvillous or crypt ORNs for bile salt detection, a strategy thus far undocumented in fishes. It is currently unknown which receptor types agnathans use to detect particular odorant classes, since it was only recently discovered that lampreys possess three ORN types similar to those in the teleost OE (Laframboise et al., 2007). In teleosts, ORN type and function are correlated (Hansen et al., 2003), so it is puzzling that elasmobranchs detect a comparable suite of odorants with fewer types of ORNs. One possible explanation is that elasmobranchs possess multiple sub-types of microvillous and crypt ORNs with at least somewhat distinct odorant specificities. In their investigation of the G–protein associations of morphologically different ORNs in teleosts, Hansen and Zielinski (2005) reported the presence of three sub-classes of microvillous ORNs, each coupled to a specific G-protein αsubunit. It is possible that multiple sub-classes of microvillous ORNs exist in the elasmobranch OE and that at least one type mediates responses to bile salt odorants. The two elasmobranch species tested demonstrated smaller relative EOG responses (Fig. 3.3) and lower sensitivity (Table 3.1) to bile salts compared to teleosts and agnathans. For D. sabina and S. tiburo, the olfactory thresholds to the four bile salts ranged from 10 -7.2 -5.5 to 10 -1 mol l . These thresholds occur at the high end of the range for teleost fishes, which exhibit olfactory thresholds to bile salts between 10 -12 -6 -1 to 10 mol l (Hara, 1994; Huertas et al., 2010; Michel and Lubomudrov, 1995; Zhang and Hara, 2009), and are notably higher than thresholds estimated for agnathans, which reach the sub-picomolar range (Li et al., 1995; Siefkes and Li, 2004; Sorensen et al., 2005). With respect to bile salts being biologically relevant to fishes, it is rather interesting to note that bile salts are also detected by the taste system of fishes with an estimated threshold to taurocholic acid in rainbow trout in the picomolar range (Yamashita et al., 2006) and for the channel catfish taste system to bile salts in the 10-100 picomolar range (Rolen 65 and Caprio, 2008). Unfortunately, little is currently known concerning the physiology of the gustatory system in any elasmobranch. A potential reason for the relatively small response magnitudes and limited sensitivities reported here for the olfactory system of elasmobranchs is that the bile salts tested are produced by teleosts (Zhang and Hara, 2009). Cholic acid is one of the most commonly produced bile salts in teleost fishes (Hagey et al., 2010; Haslewood, 1967). If elasmobranchs use C24 bile salts to localize teleost prey, one would predict a high olfactory sensitivity to these compounds, such as CA; our results do not support this. Teleosts are thought to potentially use bile salts produced by conspecifics as pheromones (Sorensen and Caprio, 1997; Sorensen and Stacey, 2004; Zhang et al., 2001). If this occurs in elasmobranchs as well, then bile salts that are produced by elasmobranchs, such as 5β-scymnol 27-sulfate, might prove more potent as an olfactory stimulus. Unfortunately, these elasmobranch-produced bile salts are currently commercially unavailable, so they must either be isolated or synthesized in order to be used in future olfactory studies. Overall, the two elasmobranch species in this study demonstrated comparable EOG responses to the bile salts; however, some interspecific differences between the two species were evident. The EOG responses of D. sabina to the four bile salts were consistently of greater relative magnitude than those of S. tiburo (Fig. 3.3). This is contrary to a trend we previously reported where the EOG responses of two shark species to amino acids were approximately three times greater than those of three batoid species (skates and rays), suggesting that this trend is odorant dependent (Meredith and Kajiura, 2010). Significantly lower olfactory thresholds -7.2 (10 -6.9 and 10 -1 mol l ) to the four tested bile salt odorants were observed for Dasyatis sabina than for S. tiburo (10 -5.5 -6.5 to 10 -1 mol l ) (Table 3.1). The bile salt olfactory thresholds reported here are notably higher than those reported for the same two species to amino acids (10 7.3 -1 -8.4 mol l thresholds for D. sabina and 10 -7.3 to 10 -8.1 to 10 -1 mol l thresholds for S. tiburo) (Meredith and Kajiura, 2010). Although the relative magnitude of the responses of both species to all four bile salts were within 11% of each other, cholic acid was the least stimulatory with the highest olfactory 66 - threshold for both species. In addition, the olfactory responsivity for D. sabina to CA across multiple stimulus concentrations is less than those to the other three bile salts (Fig. 3.4). This may reflect the differences in amidation between CA and the other three bile salts; free bile salts (non-conjugated) also elicited smaller maximum responses from the olfactory system of lake char (Salvelinus namaycush) when compared to amidated bile salts (conjugated with either taurine or glycine) (Zhang and Hara, 2009). The distinct relative effectiveness and concentration-response curves between free and amidated bile salts seen here may suggest that elasmobranchs detect them using distinct molecular OR types. Teleosts and agnathans distinguish among bile salts depending on amidation and the type and position of the conjugating group (Li and Sorensen, 1997; Michel and Derbidge, 1997; Rolen and Caprio, 2007; Zhang and Hara, 2009). Between three and six bile salt ORs were characterized for teleosts out of the ~100 molecular ORs which have been identified (Ngai and Alioto, 2008). Cross-adaptation or mixture experiments that test bile salts from different groups against each other would elucidate this possibility. While we cannot conclusively confirm whether elasmobranchs possess multiple OR types that distinguish among bile salt odorants, our cross-adaptation data (Fig. 3.5) demonstrated that amino acid and bile salt odorants interact with independent molecular ORs in the OE of both elasmobranch species. Though the mean PURs were occasionally >100% due to the small sample size and inter-individual variability, overall the mean PURs were near 100% under all amino acid adapting regimes (89% for D. sabina and 102% for S. tiburo). Thus, the continuous presence of an amino acid at the OE had little effect on the responses to bile salts, indicating that these two groups of odorants bind to relatively independent molecular ORs. Teleost fishes also possess independent OR populations that distinguish between bile salt and amino acid odorants (Michel and Derbidge, 1997; Zhang and Hara, 2009), though it is currently unknown whether this also occurs in agnathans. Amino acids and bile salts have distinct molecular structures which activate different ORs in both elasmobranch and teleost fishes. Even though they lack the seemingly necessary ciliated ORN type in their OE, elasmobranchs are able to distinguish bile salts from other odorants with a high degree of specificity. For most vertebrates, including 67 teleosts, the axons of ORNs expressing different classes of ORs converge onto separate regions in the olfactory bulb (OB) (Friedrich and Korsching, 1998; Hamdani and Døving, 2007; Nikonov and Caprio, 2001; Xu et al., 2000). It is unknown whether elasmobranchs, a phylogenetically basal vertebrate group of fishes, possess a similar chemotopic OB map. Future studies should test the responses of elasmobranchs to elasmobranch-produced bile salts, investigate which ORN type(s) expresses molecular ORs that detect bile salts, and determine whether the responses to bile salt stimuli are chemotopically mapped onto the elasmobranch OB. 68 REFERENCES Ache, B. W. and Young, J. M. (2005). Olfaction: Diverse Species, Conserved Principles. Neuron 48, 417-430. Bridgwater, R. J., Haslewood, G. A. D. and Briggs, T. (1962). Comparative Studies of 'Bile Salts' .14. Isolation from Shark Bile and Partial Synthesis of Scymnol. Biochem. J. 82, 285-290. Caprio, J. and Byrd, R. P. (1984). Electrophysiological Evidence for Acidic, Basic, and Neutral Amino-Acid Olfactory Receptor-Sites in the Catfish. J. Gen. Physiol. 84, 403-422. Compagno, L. J. V. (2002). Sharks. In The Living Marine Resources of the Western Central Atlantic, vol. 1: Introduction, Molluscs, Crustaceans, Hagfishes, Sharks, Batoid Fishes and Chimaeras (ed. K. E. Carpenter), pp. 357-505. Rome, Italy: FAO. Denton, J. E., Yousef, M. K., Yousef, I. M. and Kuksis, A. (1974). Bile Acid Composition of Rainbow Trout, Salmo gairdneri. Lipids 9, 945-951. Døving, K. B., Hansson, K. A., Backstrom, T. and Hamdani, E. H. (2011). Visualizing a set of olfactory sensory neurons responding to a bile salt. J. Exp. Biol. 214, 80-87. Døving, K. B., Selset, R. and Thommesen, G. (1980). Olfactory Sensitivity to Bile-Acids in Salmonid Fishes. Acta Physiol. Scand. 108, 123-131. Eisthen, H. L. (2002). Why Are Olfactory Systems of Different Animals So Similar? Brain, Behavior & Evolution 59, 273-293. Eisthen, H. L. (2004). The goldfish knows: Olfactory receptor cell morphology predicts receptor gene expression. J. Comp. Neurol. 477, 341-346. Ferrando, S., Bottaro, M., Gallus, L., Girosi, L., Vacchi, M. and Tagliafierro, G. (2006). Observations of crypt neuron-like cells in the olfactory epithelium of a cartilaginous fish. Neurosci. Lett. 403, 280-282. Ferrando, S., Gambardella, C., Ravera, S., Bottero, S., Ferrando, T., Gallus, L., Manno, V., Salati, A. P., Ramoino, P. and Tagliafierro, G. (2009). Immunolocalization of G-Protein Alpha Subunits in the Olfactory System of the Cartilaginous Fish Scyliorhinus Canicula. The Anatomical Record 292, 1771-1779. 69 Friedrich, R. W. and Korsching, S. I. (1998). Chemotopic, Combinatorial, and Noncombinatorial Odorant Representations in the Olfactory Bulb Revealed Using a Voltage-Sensitive Axon Tracer. J. Neurosci. 18, 9977-9988. Hagey, L. R., MØller, P. R., Hofmann, A. F. and Krasowski, M. D. (2010). Diversity of Bile Salts in Fish and Amphibians: Evolution of a Complex Biochemical Pathway. Physiol. Biochem. Zool. 83, 308-321. Hamdani, E. H. and Døving, K. B. (2007). The functional organization of the fish olfactory system. Prog. Neurobiol. 82, 80-86. Hansen, A., Anderson, K. T. and Finger, T. E. (2004). Differential distribution of olfactory receptor neurons in goldfish: Structural and molecular correlates. J. Comp. Neurol. 477, 347-359. Hansen, A. and Finger, T. E. (2000). Phyletic distribution of crypt-type olfactory receptor neurons in fishes. Brain. Behav. Evol. 55, 100-10. Hansen, A., Rolen, S. H., Anderson, K., Morita, Y., Caprio, J. and Finger, T. E. (2003). Correlation between Olfactory Receptor Cell Type and Function in the Channel Catfish. The J.Neurosci. 23, 9328-9339. Hansen, A., Rolen, S. H., Anderson, K., Morita, Y., Caprio, J. and Finger, T. E. (2005). Olfactory Receptor Neurons in Fish: Structural, Molecular and Functional Correlates. Chem. Senses 30, i311. Hansen, A. and Zielinski, B. (2005). Diversity in the olfactory epithelium of bony fishes: Development, lamellar arrangement, sensory neuron cell types and transduction components. J. Neurocytol. 34, 183-208. Hara, T. J. (1994). The diversity of chemical stimulation in fish olfaction and gustation. Rev. Fish Biol. Fisher. 4, 1-35. Haslewood, G. A. D. (1967). Bile salt evolution. J. Lipid Res. 8, 535-550. Hofmann, A. F., Hagey, L. R. and Krasowski, M. D. (2010). Bile salts of vertebrates: structural variation and possible evolutionary significance. J. Lipid Res. 51, 226-246. 70 Huertas, M., Hagey, L., Hofmann, A. F., Cerdà, J., Canário, A. V. M. and Hubbard, P. C. (2010). Olfactory sensitivity to bile fluid and bile salts in the European eel (Anguilla anguilla), goldfish (Carassius auratus) and Mozambique tilapia (Oreochromis mossambicus) suggests a 'broad range' sensitivity not confined to those produced by conspecifics alone. J. Exp. Biol. 213, 308-317. Huertas, M., Hubbard, P. C., Canário, A. V. M. and Cerdà, J. (2007). Olfactory sensitivity to conspecific bile fluid and skin mucus in the European eel Anguilla anguilla (L.). J Fish Biol. 70, 1907-1920. Laberge, F. and Hara, T. J. (2004). Electrophysiological demonstration of independent olfactory receptor types and associated neuronal responses in the trout olfactory bulb. Comp. Biochem. Physiol. 137A, 397-408. Laframboise, A. J., Ren, X., Chang, S., Dubuc, R. and Zielinski, B. S. (2007). Olfactory sensory neurons in the sea lamprey display polymorphisms. Neurosci. Lett. 414, 277-281. Li, W., Scott, A. P., Siefkes, M. J., Yan, H., Liu, Q., Yun, S.-S. and Gage, D. A. (2002). Bile Acid Secreted by Male Sea Lamprey That Acts as a Sex Pheromone. Science 296, 138. Li, W., Sorensen, P. and Gallaher, D. (1995). The olfactory system of migratory adult sea lamprey (Petromyzon marinus) is specifically and acutely sensitive to unique bile acids released by conspecific larvae. J. Gen. Physiol. 105, 569-587. Li, W. and Sorensen, P. W. (1997). Highly independent olfactory receptor sites for naturally occurring bile acids in the sea lamprey, Petromyzon marinus. J. Comp. Physiol. A. 180, 429-438. Lipschitz, D. L. and Michel, W. C. (2002). Amino Acid Odorants Stimulate Microvillar Sensory Neurons. Chem. Senses 27, 277-286. McEachran, J. D. and de Carvalho, M. R. (2002). Batoid Fishes. In The Living Marine Resources of the Western Central Atlantic, vol. 1: Introduction, Molluscs, Crustaceans, Hagfishes, Sharks, Batoid Fishes and Chimaeras (ed. K. E. Carpenter), pp. 507–589. Rome, Italy: FAO. 71 Meredith, T. L. and Kajiura, S. M. (2010). Olfactory morphology and physiology of elasmobranchs. J. Exp. Biol. 213, 3449-3456. Michel, W. C. and Derbidge, D. S. (1997). Evidence of distinct amino acid and bile salt receptors in the olfactory system of the zebrafish, Danio rerio. Brain Res. 764, 179-187. Michel, W. C. and Lubomudrov, L. M. (1995). Specificity and Sensitivity of the Olfactory Organ of the Zebrafish, Danio rerio. J. Comp. Physiol. A. 177, 191-199. Ngai, J. and Alioto, T. S. (2008). Genomics of Odor Receptors in Zebrafish. In The Senses: A Comprehensive Reference, vol. 4, Olfaction & Taste eds. A. I. Basbaum K. Akimichi G. M. Shepherd and G. Westheimer), pp. 553-560. San Diego, CA: Academic Press. Nikonov, A. A. and Caprio, J. (2001). Electrophysiological evidence for a chemotopy of biologically relevant odors in the olfactory bulb of the channel catfish. J. Neurophysiol. 86, 1869-1876. Rolen, S. H. and Caprio, J. (2007). Processing of bile salt odor information by single olfactory bulb neurons in the channel catfish. J. Neurophysiol. 97, 4058-4068. Rolen, S. H. and Caprio, J. (2008). Bile salts are effective taste stimuli in channel catfish. J. Exp. Biol. 211, 2786-2791. Rolen, S. H., Sorensen, P. W., Mattson, D. and Caprio, J. (2003). Polyamines as olfactory stimuli in the goldfish Carassius auratus. J. Exp. Biol. 206, 1683-1696. Sato, K. and Suzuki, N. (2001). Whole-cell response characteristics of ciliated and microvillous olfactory receptor neurons to amino acids, pheromone candidates and urine in rainbow trout. Chem. Senses 26, 1145-1156. Schluessel, V., Bennett, M. B., Bleckmann, H., Blomberg, S. and Collin, S. P. (2008). Morphometric and ultrastructural comparison of the olfactory system in elasmobranchs: the significance of structure-function relationships based on phylogeny and ecology. J. Morphol. 269, 1365-1386. 72 Siefkes, M. J. and Li, W. (2004). Electrophysiological evidence for detection and discrimination of pheromonal bile acids by the olfactory epithelium of female sea lampreys (Petromyzon marinus). J. Comp. Physiol. A. 190, 193-199. Silver, W. L. (1979). Olfactory responses from a marine elasmobranch, the Atlantic stingray, Dasyatis sabina. Mar. Behav. Physiol. 6, 297-305. Sorensen, P. W. and Caprio, J. (1997). Chemoreception. In The Physiology of Fishes, (ed. D. H. Evans), pp. 375-405. Boca Raton, FL: CRC Press. Sorensen, P. W., Fine, J. M., Dvornikovs, V., Jeffrey, C. S., Shao, F., Wang, J., Vrieze, L. A., Anderson, K. R. and Hoye, T. R. (2005). Mixture of new sulfated steroids functions as a migratory pheromone in the sea lamprey. Nat. Chem. Biol. 1, 324-328. Sorensen, P. W. and Stacey, N. E. (2004). Brief review of fish pheromones and discussion of their possible uses in the control of non-indigenous teleost fishes. N. Z. J. Mar. Freshwater Res. 38, 399-417. Takami, S., Luer, C. A. and Graziadei, P. C. (1994). Microscopic structre of the olfactory organ of the clearnose skate, Raja eglanteria. Anat. Embryol. 190, 211-230. Tricas, T. C., Kajiura, S. M. and Summers, A. P. (2009). Response of the hammerhead shark olfactory epithelium to amino acid stimuli. J. Comp. Physiol. A. 195, 947-954. Velez, Z., Hubbard, P. C., Welham, K., Hardege, J. D., Barata, E. N. and Canario, A. V. M. (2009). Identification, release and olfactory detection of bile salts in the intestinal fluid of the Senegalese sole (Solea senegalensis). J. Comp. Physiol. A. 195, 691-698. Vielma, A., Ardiles, A., Delgado, L. and Schmachtenberg, O. (2008). The elusive crypt olfactory receptor neuron: evidence for its stimulation by amino acids and cAMP pathway agonists. J. Exp. Biol. 211, 2417-2422. Xu, F. Q., Greer, C. A. and Shepherd, G. M. (2000). Odor maps in the olfactory bulb. J. Comp. Neurol. 422, 489-495. 73 Yamashita, S., Yamada, T. and Hara, T. J. (2006). Gustatory responses to feeding and nonfeeding-stimulant chemicals, with an emphasis on amino acids, in rainbow trout. J. Fish Biol. 68, 783-800. Zeiske, E., Caprio, J. and Gruber, S. H. (1986). Morphological and electrophysiological studies on the olfactory organ of the lemon shark, Negaprion brevirostris. In Indo-Pacific Fish Biology: Proceedings of the Second International Conference on Indo-Pacific Fishes, eds. T. Uyeno R. Arai T. Taniuchi and K. Matsuura), pp. 381-391. Tokyo: Ichtyological Society of Japan. Zeiske, E., Kasumyan, A., Bartsch, P. and Hansen, A. (2003). Early development of the olfactory organ in sturgeons of the genus Acipenser: a comparative and electron microscopic study. Anat. Embryol. 206, 357-372. Zeiske, E., Theisen, B. and Gruber, S. H. (1987). Functional morphology of the olfactory organ of two carcharhinid shark species. Can. J. Zool. 65, 2406-2412. Zhang, C. and Hara, T. J. (2009). Lake char (Salvelinus namaycush) olfactory neurons are highly sensitive and specific to bile acids. J. Comp. Physiol. A. 195, 203-215. Zhang, C. B., Brown, S. B. and Hara, T. J. (2001). Biochemical and physiological evidence that bile acids produced and released by lake char (Salvelinus namaycush) function as chemical signals. J. Comp. Physiol. B. 171, 161-171. Zielinski, B. and Hara, T. J. (2006). Olfaction. In Fish Physiology: Sensory Systems Neuroscience, vol. 25, pp. 1-43. San Diego, CA: Academic Press. 74 x Table 3.1 Mean olfactory thresholds (10 M ± s.e.m.) to four bile salts after compensation for stimulus dilution. Note: TLCS taurolithocholic acid 3-sulfate, TCDC taurochenodeoxycholic acid, GCDC glycochenodeoxycholic acid, CA cholic acid. 75 Figure 3.1 Experimental apparatus used to record the electro-olfactogram (EOG) during crossadaptation experiments. Animals were secured to a platform in an experimental tank filled with seawater (SW). One arm of a PVC manifold delivered ventilatory SW flow over the animal„s gills, and a second manifold arm supplied the tank with SW. A small pump delivered a constant flow of either SW or adapting amino acid (AA) solution through a flow meter and to an odor delivery pipette (ODP). Bile salt stimuli were injected into the tubing immediately above the ODP and transported to the olfactory organ. Paired, non-polarizable, Ag-AgCl electrodes recorded the EOG; the glass tip of the active electrode (Act E, inset) was positioned in the SW immediately above the olfactory epithelium, whereas the glass tip of the reference electrode (Ref E, inset) contacted the skin. The output was differentially amplified (1000x), filtered (high pass 0.1 Hz, low pass 0.1 kHz, 50/60 Hz), digitized (1 kHz) and recorded. 76 Figure 3.2 Protocol for the cross-adaptation experiments. EOG responses were recorded to four bile salts, representing nonconjugated (NC), glycine-conjugated (GC), and taurine-conjugated (TC) groups (A.), delivered individually over a background flow of seawater (SW) (B.). This pulse of bile salt odorant elicits a phasic EOG response (C.). The background flow was then randomly switched to one of four adapting amino acids, each representing a side-chain structural group. The adapting amino acid was presented continuously over the olfactory epithelium for ten minutes resulting in a tonic EOG response during which phasic responses to the bile salts were recorded. This phasic response is only seen if the test stimulus interacts with independent receptors from those for the adapting stimulus. The background flow was returned to SW for thirty minutes to allow the olfactory receptors to unadapt; and the EOG responses to the bile salts were again recorded to confirm that the animal was still responding at the pre-adaptation level. This procedure was repeated for each of the three remaining adapting regimes. The molecular structures for each bile salt, modified from Zhang and Hara (2009), are provided to the left. TLCS taurolithocholic acid 3-sulfate, TCDC taurochenodeoxycholic acid, GCDC glycochenodeoxycholic acid, CA cholic acid. 77 Figure 3.3 Mean EOG responses (+ s.d.) for D. sabina (n≥5, black bars) and S. tiburo (n=4, white -4 bars) to four bile salts at an injection concentration of 10 M and two controls, SW and 0.1% -3 MeOH. Response magnitudes are expressed as a percent of the response to the standard (10 M alanine). Letters within each bar represent the results of pair-wise comparisons using HolmSidak tests. The mean EOG response magnitudes to the four bile salts varied significantly for both species and ranged from 7-18% of the response to the standard. For each species, bile salts that share a letter do not differ significantly from each other. TLCS taurolithocholic acid 3sulfate, TCDC taurochenodeoxycholic acid, GCDC glycochenodeoxycholic acid, CA cholic acid. 78 Figure 3.4 Concentration-response relationships of four bile salts for D. sabina and S. tiburo. Response magnitudes represent the mean response for each bile salt at each concentration and -3 are expressed as a percent of the standard (10 M alanine). The EOG response increased predictably with increasing bile salt concentration. Line drawing of D. sabina is modified from McEachran and de Carvalho (2002), and line drawing of S. tiburo is modified from Compagno (2002). TLCS taurolithocholic acid 3-sulfate, TCDC taurochenodeoxycholic acid, GCDC glycochenodeoxycholic acid, CA cholic acid. 79 Figure 3.5 Mean percent unadapted responses (PUR) (+ S.E.) for D. sabina and S. tiburo to four bile salts presented individually to the olfactory epithelium during each of four L-amino acid adapting regimes. Percent unadapted responses did not significantly differ among bile salts or among amino acid adapting regimes for either species. The dotted line indicates 100% PUR. Line drawing of D. sabina is modified from McEachran and de Carvalho (2002), and line drawing of S. tiburo is modified from Compagno (2002). TLCS taurolithocholic acid 3-sulfate, TCDC taurochenodeoxycholic acid, GCDC glycochenodeoxycholic acid, CA cholic acid. 80 CHAPTER 4 THE SOMATOTOPIC ORGANIZATION OF THE HEMI-OLFACTORY BULB IN ELASMOBRANCHS ABSTRACT Odorant detection in vertebrates occurs when odorants bind to molecular olfactory receptors located on olfactory receptors neurons (ORNs) in the olfactory epithelium (OE). The axons of the ORNs converge to form cranial nerve I (the olfactory nerve) and project to the olfactory bulb (OB) of the forebrain where they synapse with mitral cells in a bundle of neuropil, termed glomeruli. Several elasmobranch (sharks, rays, and skates) species possess OBs that are each physically subdivided into two or more “hemi-olfactory bulbs”; this OB morphology has not been documented in any other vertebrate. The functional significance of this unique morphology is not fully understood, but may reflect either a chemotopic or somatotopic segregation of ORN projections. Virtually all vertebrate OBs are arranged chemotopically; however, a previous study with elasmobranchs suggested somatotopy instead. The present study examined the morphology and organization of ORN projections from the OE to the OB in three elasmobranchs species with varying OB morphologies using histological staining and retrograde tracing techniques. In all three species glomeruli in the OB received projections from ORNs located on the only three to five lamellae situated immediately anterior. These results suggest that the elasmobranch OB is arranged somatotopically rather than chemotopically, like that of teleost fishes and most other vertebrates. INTRODUCTION Chemical sensitivity is present in nearly all life forms, from bacteria to mammals, and plays an integral role for many of these organisms in feeding, reproduction, and predator 81 detection (Ache and Young, 2005; Firestein, 2001). A remarkable degree of convergence occurs in the organization of the olfactory pathways in a phylogenetically diverse array of animals, including the use of G-protein coupled receptors for odorant detection, perireceptor processes that affect odorant detection (e.g. sniffing), and the olfactory neuronal circuitry (Ache and Young, 2005; Firestein, 2001). Animal models are useful not only in investigating olfaction as it applies to more derived vertebrates, but also in understanding the evolution of this sensory modality. Elasmobranch fishes (sharks, rays, and skates) are an excellent model for the study of olfaction for several reasons. They have persisted in the vertebrate clade for nearly 450 million years (Janvier, 1996) and possess interspecific diversity in the morphology of their olfactory systems. Additionally, the ~800 extant elasmobranch species occupy a wide variety of ecological niches that impose diverse selective pressures on this chemosensory modality. Despite the advantages of studying olfaction in elasmobranchs, many assumptions have been drawn about the elasmobranch olfactory system based on information in the teleost literature. Odorant detection in teleosts begins when chemical-laden water passes through the incurrent naris into the olfactory capsule, which houses the peripheral olfactory organ. This olfactory organ is comprised of plate-like lamellae overlain with the olfactory epithelium (OE), which contains millions of olfactory receptors neurons (ORNs) (Yamamoto, 1982). Odorant molecules bind to molecular olfactory receptors (ORs) on the cilia or microvilli of the bipolar ORNs, whose axons converge beneath the OE in the lamina propria of the lamellae. These axon bundles form the olfactory nerve and project to the olfactory bulb (OB) of the brain where they synapse with mitral cells in a spherical bundle of axons and dendrites, termed glomeruli (Baier and Korsching, 1994; Baier et al., 1994; Banniste, 1965; Byrd and Brunjes, 1995; Hansen and Zeiske, 1998; Laberge and Hara, 2001; Zielinski and Hara, 2006). In teleost fishes, each ORN expresses a single molecular OR type making them sensitive to a particular group of odorants. ORNs expressing a given OR type are randomly distributed throughout the sensory portion of the OE; however their axons converge and project to spatially distinct regions in the OB (Baier et al., 1994; Friedrich and Korsching, 1997; Ngai et al., 1993). 82 Both axonal tracing and electro-physiological studies have confirmed that the teleost OB is divided into separate functional zones that process different types of odorants (Hamdani and Døving, 2007). For example, microvillous ORNs respond primarily to amino acids and their axons project to specific regions in the dorsolateral and ventral OB (Døving et al., 2011; Hansen et al., 2003; Nikonov and Caprio, 2001; Sato and Suzuki, 2001). If examined on a broader scale, the medial region of the OB seems to selectively process pheromonal information whereas the lateral OB seems to process non-pheromonal information, such as feeding cues (Hamdani and Døving, 2007; Nikonov and Caprio, 2001). This chemotopic organization of the OB, which is also found in insects, amphibians, and mammals, is thought to enhance discrimination and detection of odors and play an important role in encoding olfactory information (Caprio and Derby, 2008; Hildebrand and Shepherd, 1997; Johnson and Leon, 2007; Nikonov and Caprio, 2001; Strausfeld and Hildebrand, 1999). The elasmobranch olfactory system seems to be morphologically and physiologically similar to that of teleost fishes. Like teleosts, elasmobranchs possess different morphological types of ORNs in the OE, whose axons synapse at glomeruli within a superficial layer of the OB (Fig. 4.1C) (Hansen and Zielinski, 2005; Northcutt, 1978; Schluessel et al., 2008; Takami et al., 1994; Theisen et al., 1986). In addition, the olfactory system of elasmobranchs demonstrates a similar specificity and sensitivity to amino acid odorants as teleosts (Hara, 1994; Meredith and Kajiura, 2010; Silver, 1979; Tricas et al., 2009; Zeiske et al., 1986). However, there are notable differences in the olfactory systems of these sympatric species. For example, the OB in teleosts typically has a round shape and is often located closer to the rest of the forebrain than the olfactory organ (sessile placement); whereas the OB of elasmobranchs is an elongated structure that lies parallel and immediately adjacent to the olfactory organ (pedunculated placement) (Northcutt, 1978; Zielinski and Hara, 2006) (Fig. 4.1B). Also, in some elasmobranch species, the OB is physically partitioned into either two distinct hemi-bulbs, as exemplified in the lemon shark (Negaprion brevirostris) and the Atlantic sharpnose shark (Rhizoprionadon terraenovae), or as a succession of connected bulbar swellings, as seen in the bonnethead shark (Sphyrna tiburo) 83 (Northcutt, 1978). The number of elasmobranch species possessing some form of hemi-OB morphology is unknown; however, these morphologies are evident in brain photographs and illustrations of several species in works by Northcutt (1978) and Garman (1913). While the functional significance of this hemi-OB morphology is currently not understood, it may indicate either a functional segregation of olfactory projections from the OE to the separate hemi-OBs, similar to the medial and lateral portions of the teleost OB, or a spatial segregation of projections to maintain a spatial component in the processing of olfactory information. A study examining three elasmobranch species (Dasyatis sabina, R. terraenovae, and S. tiburo) found that ORNs in the medial half of the OE projected immediately posterior to glomeruli in the medial half of the OB, and ORNs in the lateral OE projected to the lateral OB (Dryer and Graziadei, 1993). These data suggest a somatotopic arrangement, in which sensory projections maintain their spatial organization from the peripheral to the central nervous system, as opposed to the functional, or chemotopic, arrangement seen in teleost fishes and other vertebrates. Dryer and Graziadei (1993) employed anterograde tracing (from the OE to the OB) using biocytin, a histological stain for neurons, to label the OE in two of the species (D. sabina and R. terranovae) and DiI, a lipophilic carbocyanine dye, in the OE of S. tiburo. They concluded that in the biocytinlabeled specimens, boundaries of the projections from individual lamellae were difficult to define and that DiI-labeling yielded superior definition compared to biocytin. In addition, given the limitations of their methodology, their goal was to determine the segregation of input between the medial and lateral halves of the OB, rather than to define precise projection regions. In the present study, we examined the morphology and organization of ORN projections in three elasmobranch species, D. sabina, D. say, and N. brevirostris, that possess differing OB morphologies in order to more precisely assess the extent of somatotopy in the elasmobranch OB. Specifically, we tested the hypothesis put forth by Dryer and Graziadei (1993) that axons projecting from the OE to the OB exhibit a somatotopic arrangement, which may be related to the hemi-OB morphology. 84 METHODS Sample collection We examined the morphology and organization of the olfactory epithelium (OE) and olfactory bulbs (OBs) of two stingray species and one shark species representing two elasmobranch orders: the Atlantic stingray Dasyatis sabina (Lesueur 1824) and the bluntnose stingray, D. say (Lesueur 1817) from the order Rajiformes, and the lemon shark Negaprion brevirostris (Poey 1868) from the order Carcharhiniformes (Fig. 4.1A). The two stingray species possess elongate, cohesive OBs whereas the lemon shark possesses OBs divided into two physically distinct units, or hemi-OBs (Fig. 4.1B). Adult stingray samples were acquired during the collections of the Florida Fish and Wildlife Conservation Commission in the Indian River Lagoon. Samples from juvenile lemon sharks were obtained from other investigators at the Elasmobranch Research Laboratory at Florida Atlantic University in Boca Raton, Florida. All animals were humanely euthanized, the cranial cavity carefully exposed, and the head immediately fixed by immersion in 4% paraformaldehyde in 0.1M phosphate buffer for a minimum of 48 hours. Both left and right olfactory structures were used from each animal, resulting in two samples per individual. Histological staining The overall organization and histology of the OB and OE of a single D. say sample was examined by treating cryosections (30-40 µm) with the histological stain Kernechtrot-LichtgrünOrange (KLO) (nuclear red-light green-orange). For the staining procedure, the slides containing the olfactory structures were washed in dH 2O for two minutes, immersed in a mixture of nuclear red in aluminum sulphate for ten minutes, washed in dH2O for five seconds, and immersed in a mixture of light green and orange G in phosphotungstic acid for two minutes. After the staining process, the slides were dehydrated in ethanol (96% for ten seconds, 96% for twenty seconds, 100% for five minutes, and 100% for five minutes) and xylene (twice for ten minutes each). Slides were then sealed with coverslips using Permount mounting medium (Fisher Scientific, Pittsburgh, PA) and examined under a light microscope (Olympus, Center Valley, PA). KLO 85 stains nuclei red, the collagenous connective tissue and basal lamina were stained green, erythrocytes stained yellow to orange, neuron somas appear red (due to nuclear staining), and neuropil (neuron processes) stain slightly greenish-gray (Anne Hansen, personal communication). Retrograde tracing The olfactory receptor neuron (ORN) projections from the OE to the OB of D. sabina (n=2), D. say (n=4), and N. brevirostris (n=12) were visualized using retrograde tracing techniques, which label the cell membrane of neurons without crossing synapses. Small crystals of 1,1'-dioctadecyl-3,3,3'3'-tetramethylindocarbocyanine perchlorate (DiI) and/or PTIR271 (Far Red), two lipophilic carbocyanine dyes that fluoresce at different wavelengths, were placed on the tip of a needle and inserted into various locations on the dorsal surface of the OBs of each animal. Since these dyes do not cross synapses, the only cells that should be labeled are those whose axons reside at the labeling site and came into contact with the dye. The brain was then covered in 3% agar to prevent the dye from spreading and re-immersed in 4% paraformaldehyde for a minimum of four months to allow the dye to diffuse throughout the brain and epithelium. Once the dye had ample time to travel, the olfactory organ and bulb were dissected from the head and embedded in either egg yolk or 15% gelatin. The OB and OE-containing gelatinous block was then fixed overnight in 4% paraformaldehyde. The following day, 50-150 µm sections were cut on a vibratome (PELCO, Redding, CA), mounted on slides, and examined with epifluorescence using a Zeiss microscope (Thornwood, NY) or an Olympus confocal laser scanning microscope (Center Valley, PA). RESULTS Morphology Using both KLO and carbocyanine dye-labeled samples, we examined the morphology of the OB in three elasmobranch species representing two OB morphologies (Fig. 4.1B). We observed a laminar organization of the OB in all three species with a superficial, thick fibrous layer at the posterior of the OB (Fig. 4.2). At the anterior face of the OB, adjacent to the OB/OE 86 interface is the olfactory nerve layer in which axons enter the OB from the OE and exhibit considerable divergence before synapsing at their target glomeruli (Fig. 4.3A). Deep to the fibrous and olfactory nerve layers is a wide, ill-defined glomerular layer (Figs. 4.2, 4.3A, 4.3B, 4.4B). For all three species, the glomeruli in the OB were distributed throughout and not limited to a distinct layer at the outer rim of the OB. The OE of all three species exhibited secondary folding with sensory epithelium located in the “troughs” and non-sensory epithelium in the “ridges” of the tissue folds (Figs. 4.2, 4.3C, 4.3D, 4.3F). Secondary OE folding throughout all lamellae was seen in the two Dasyatid species, whereas in the lemon shark the secondary folding was absent from the more dorsal base of the lamellae located in the middle of the olfactory organ. We found microvillous ORNs (Figs. 4.3G, 4.4D, 4.4E) and very few crypt ORNs, but no ciliated ORNs (as far as can be seen in a light microscopic study). The few crypt ORNs seen were egg-shaped and occurred in the upper half of the OE. Organization of ORN projections All three elasmobranch species, for which the precise OB labeling site varied among samples (illustrated as insets in Figures 4.3, 4.4, and 4.5), exhibited a similar pattern of ORN projections from the OE to the OB (Figs. 4.3, 4.4). Dye placement in the OB resulted in labeling of the glomeruli and axons in the OB near the labeling site. The adjacent olfactory nerve fascicles and axon bundles in the lamina propria of the three to five lamellae immediately anterior of the OB labeling site were retrogradely labeled (Figs. 4.3A-4.3C, 4.4A-4.4C). Labeling extended from those axon bundles into individual axons innervating the ORNs in the OE (Figs. 4.3D-4.3G, 4.4D, 4.4E). No labeling was ever seen in lamellae distant from the labeling site (i.e. ORNs project only to a very limited area in the OB). In samples where DiI and Far Red were placed in separate hemi-OBs, distinct labeling of the hemi-bulbs (Fig. 4.4A), axons, lamellae, and ORNs in the OE were always observed. In samples where both DiI and Far Red were placed in two locations within the same hemi-OB, we usually observed labeling of distinct glomeruli and axons traced retrogradely into separate lamellae by each dye (Figs. 4.4D, 4.5A). Only when the two dyes were inserted into the OB in such close proximity that the same axons were affected by 87 both dyes, did we observe double labeling of axons in the OB, indicated by a yellow hue (Fig. 4.5B). DISCUSSION The hemi-olfactory bulb morphology found in numerous elasmobranch species, like the lemon shark, is undocumented in other organisms (Northcutt, 1978; Tester, 1963). The pervasiveness of this morphology in elasmobranch clades and how it relates to the processing of odor information is not fully understood, but it may indicate either a functional segregation of olfactory projections from the OE to separate hemi-OBs, similar to teleosts (Hamdani and Døving, 2007), or a spatial segregation of projections to maintain a spatial component in the processing of olfactory information. In this study, we examined the morphology of the olfactory structures and organization of the ORN projections in three elasmobranch species with varying OB morphologies to test the hypothesis that axons projecting from the OE to the OB exhibit a somatotopic, or spatial, arrangement. We found in all three species that glomeruli, which were distributed throughout the OB (Figs. 4.2, 4.3A, 4.3B, 4.4B), only received projections from ORNs located on the three to five lamellae situated immediately anterior (Figs. 4.3, 4.4). These results support our hypothesis and previous results from Dryer and Graziadei (1993) that the OB of elasmobranchs is unique in that it is arranged somatotopically rather than chemotopically, like that of teleost fishes and most other vertebrates. Morphology To understand the arrangement of the elasmobranch OB, we first examined the gross morphology and fine structure of the olfactory system. The OB of elasmobranchs exhibits a pedunculated placement (Fig. 4.1B) in contrast to the sessile OB placement of many teleost species (Northcutt, 1978; Zielinski and Hara, 2006). The teleost OB is divided into four distinct concentric layers: from most superficial to deep there is the olfactory nerve layer, the glomerular layer, the mitral cell layer, and the granule cell layer (Byrd and Brunjes, 1995; Laberge and Hara, 2001; Oka et al., 1982). The cytoarchitecture of the OB for all three species was similar to that previously described for teleosts and other elasmobranch species (Dryer and Graziadei, 1993; 88 Ferrando et al., 2009; Takami et al., 1994; Tester, 1963). We observed a superficial fibrous layer in the posterior OB; at the anterior face of the OB, adjacent to the OB/OE interface, was the olfactory nerve layer in which axons enter from the OE. Deep to that layer was a wide, poorly defined glomerular layer. Both the clearnose skate, Raja eglanteria, and the small-spotted catshark, Scyliorhinus canicula, possess a glomerular layer where mitral cells were situated among the glomeruli; therefore, these two layers (glomerular and mitral cell layers) are indistinguishable (Ferrando et al., 2009; Takami et al., 1994). The deepest OB layer described for elasmobranchs is the granule cell layer, which was not visibly defined with the staining methods used in this study. Although the elasmobranch OB demonstrates many morphological similarities with the teleost OB, two differences are apparent. The glomerular layer in the teleost OB is thinner and much more distinct than that in elasmobranchs, and the glomerular layer in elasmobranchs may be interspersed with the mitral cell layer instead of occurring as two distinct layers, as seen in teleosts (Baier and Korsching, 1994; Baier et al., 1994; Byrd and Brunjes, 1995; Satou, 1992). In the OE, we noted differences in the degree of secondary folding between the two Dasyatid species and the lemon shark. In their survey of elasmobranch olfactory morphology, Schluessel et al. found secondary folding of the olfactory epithelium in all 21 species examined and reported considerable differences in the degree of folding among them (2008). The two Dasyatid species in our study exhibited secondary OE folding throughout all lamellae, whereas for the lemon shark the secondary folding was absent toward the more dorsal base of the lamellae that are located in the middle of the olfactory organ. In the clearnose skate and the brownbanded bamboo shark (Chiloscyllium punctatum) the inverse scenario to the lemon shark seems to occur; the secondary folds are present over most of the OE but disappear toward their ventral free margin (Schluessel et al., 2008; Takami et al., 1994). The OE of all three species examined here exhibited secondary folds of OE with sensory epithelium located in the “troughs” and non-sensory epithelium in the “peaks” of the tissue folds, similar to other elasmobranch species (Takami et al., 1994; Tester, 1963; Theisen et al., 1986). In contrast, the sensory 89 epithelium of the spiny dogfish (Squlaus acanthius) and the small-spotted catshark was found on both the ridges and the troughs of the secondary epithelial folds (Theisen et al., 1986). The Port Jackson shark, Heterodontus portusjacksoni, demonstrated an irregular and patchy arrangement of sensory and nonsensory epithelium within the OE (Schluessel et al., 2008). This suggests that interspecific variation exists not only in the degree of secondary folding of the OE but also the precise location of the sensory epithelium within those folds. Nearly all studies on the types of ORNs present in the elasmobranch sensory OE, including this study, have confirmed the presence of microvillous ORNs (Bronshtein, 1976; Reese and Brightman, 1970; Schluessel et al., 2008; Takami et al., 1994; Theisen et al., 1986). Ciliated ORNs found in the OE of teleost fishes and many other vertebrate groups, including humans, seem to be lacking in the elasmobranch OE (Eisthen, 2004; Schluessel et al., 2008; Theisen et al., 1986). We did not observe any ciliated ORNs in the species examined here. A third ORN type, the crypt ORN, which occurs in the OE of lampreys and actinopterygian fishes, but not sarcoptergygian fishes (Hansen and Finger, 2000; Laframboise et al., 2007; Zeiske et al., 2003) was recently described in elasmobranchs (Ferrando et al., 2006). In the species investigated in this study, we observed only a few crypt ORNs. Ferrando suggested that crypt ORNs in the small-spotted catshark may project to the ventral OB (Ferrando et al., 2009). In this study, we only labeled the dorsal surface of the OB, and thus may have missed labeling the majority of crypt ORN axons. More directed studies on the projections of elasmobranch crypt ORNs are needed to confirm this hypothesis. Morphologically different ORN types are thought to mediate an animal‟s responses to specific odorant classes. Amino acids, a feeding stimulant to fishes (Zielinski and Hara, 2006), are detected primarily by microvillous ORNs (Lipschitz and Michel, 2002; Sato and Suzuki, 2001) but also possibly by ciliated and crypt ORNs (Hansen et al., 2004; Vielma et al., 2008); whereas in teleosts, bile salts are detected only by ciliated ORNs (Døving et al., 2011; Hansen et al., 2003; Sato and Suzuki, 2001). Due to the lack of ciliated ORNs in the elasmobranch OE, it was unknown whether they are able to detect bile salts, though the biological relevance of this odorant 90 class as a potential pheromone for agnathans and teleosts makes it seem likely (Døving et al., 1980; Li et al., 2002; Siefkes and Li, 2004; Sorensen et al., 2005; Sorensen and Stacey, 2004; Zhang et al., 2001). In a recent electrophysiological study, we demonstrated that the olfactory system of two elasmobranch species (Dasyatis sabina and Sphyrna tiburo) responds to bile salt odorants, though they must use a different ORN type than teleost fishes (Meredith et al., In prep). Organization of ORN projections In addition to examining the cytoarchitecture of the elasmobranch olfactory structures, we also investigated the organization of projections of the ORNs from the OE to the OB. Two of the first studies to describe the general organization of projections to the OB in elasmobranchs drew differing conclusions (Daniel, 1934; Norris and Hughes, 1920). Norris and Hughes (1920) found that the medial part of the OB received input from the medial and lateral OE, whereas the lateral OB received input only from the lateral OE. In contrast, Daniel (1934) concluded that the medial and lateral portions of the OB received input from the medial and lateral portions of the OE respectively. A more recent study (Dryer and Graziadei, 1993) using anterograde tracing techniques confirmed the results of Daniel (1934), demonstrating that ORNs in the medial and lateral halves of the OE projected immediately posterior to glomeruli in the medial and lateral halves of the OB respectively, though due to limitations of their methodology they could not define precise boundaries of the projections from individual lamellae. These results coupled with those of Daniel (1934) suggest a somatotopic arrangement in which sensory projections maintain their spatial organization in the CNS. However, in a 2009 study examining the immunolocalization of G-protein α-subunits in the small-spotted catshark olfactory system, the authors suggested that based on the pattern of immunoreactivity present in the OB, perhaps a topographic organization exists similar to that in teleosts (Ferrando et al., 2009). Our study supports the results of Dryer and Graziadei (1993) and Daniel (1934); in all three species, we found that glomeruli only received projections from ORNs located on the three to five lamellae situated immediately anterior indicating a somatotopic OB arrangement. 91 In teleost fishes, ORNs widely distributed over the epithelium converge to individual glomeruli in the OB (Baier et al., 1994; Hamdani and Døving, 2007; Riddle and Oakley, 1991), which is divided into separate functional zones that process different types of odorants (Nikonov and Caprio, 2001; Nikonov and Caprio, 2004). This demonstrates that teleost fishes, along with most other vertebrates, exhibit a functional or chemotopic OB organization where axons converge based on OR type and project to spatially distinct regions in the OB (Baier et al., 1994; Hamdani and Døving, 2007; Nikonov and Caprio, 2001). This chemotopic organization of the OB is thought to play a significant role in encoding olfactory information (Caprio and Derby, 2008; Hildebrand and Shepherd, 1997; Johnson and Leon, 2007; Nikonov and Caprio, 2001; Strausfeld and Hildebrand, 1999). The somatotopic OB organization found in the elasmobranch species examined here and previously (Dryer and Graziadei, 1993) seems to be unique among vertebrate olfactory systems, and may be related to the hemi-olfactory bulb morphology. The functional significance of this hemi-OB morphology is not understood since no physiological studies have been undertaken to map the responses of ORNs in the OB, but it may indicate either a functional segregation of olfactory projections from the OE to separate halves or hemi-OBs, similar to the medial and lateral portions of the teleost OB, or a spatial segregation of projections to maintain a spatial component in the processing of olfactory information. The olfactory epithelium of elasmobranchs is situated in an oval, laterally elongated olfactory organ adjacent to a similarly elongate OB compared to the olfactory organ and OB of most teleost species, which is more spherical and compact. Perhaps it is too inefficient for elasmobranch ORNs on one side of the OE to synapse at distant glomeruli on the other side of the OB; and as a result, each OB effectively became two functional units that receive projections from the adjacent half of the OE. With the two halves of the OB handling an independent subset of ORNs, a physical connection between the left and right portions may not be necessary and was consequently lost in some species. Alternatively, the elasmobranch olfactory system may possess a topographical organization of ORNs in the OE based on their specificity to different odorant classes. The ORNs of teleost fishes expressing specific ORs are generally considered to 92 be randomly distributed thoughout the sensory OE; however, in mammals, ORNs with particular OR gene expression are segregated into three to four anatomically distinct zones in the OE (Ressler et al., 1993). In this scenario, ORNs would project to the OB based on both location within the OE and OR type. Future studies should examine the odorant specificities across the length of the OE to test for a potential topographic map and determine the detailed projections of the different ORN types to the OB. 93 REFERENCES Ache, B. W. and Young, J. M. (2005). Olfaction: Diverse Species, Conserved Principles. Neuron 48, 417-430. Baier, H. and Korsching, S. (1994). Olfactory Glomeruli in the Zebrafish Form an Invariant Pattern and Are Identifiable across Animals. J. Neurosci. 14, 219-230. Baier, H., Rotter, S. and Korsching, S. (1994). Connectional Topography in the Zebrafish Olfactory System - Random Positions but Regular Spacing of Sensory Neurons Projecting to an Individual Glomerulus. Proc. Natl. Acad. Sci. U. S. A. 91, 11646-11650. Banniste, L. H. (1965). Fine Structure of Olfactory Surface of Teleostean Fishes. Q. J. Microsc. Sci. 106, 333-342. Bronshtein, A. A. (1976). Some peculiarities of the fine structure of olfactory organ in elasmobranchs. ZH Evol Biokhim Fiz 12, 63-67. Byrd, C. A. and Brunjes, P. C. (1995). Organization of the Olfactory System in the Adult Zebrafish - Histological, Immunohistochemical, and Quantitative-Analysis. J. Comp. Neurol. 358, 247-259. Caprio, J. and Derby, C. D. (2008). Aquatic animal models in the study of chemoreception. In The Senses: A Comprehensive Reference, vol. 4: Olfaction and Taste eds. A. I. Basbaum A. Kaneko G. M. Shepherd and G. Westheimer), pp. 97-134. San Diego, CA: Academic Press. Daniel, J. F. (1934). The Special Senses of Heptanchus maculatus. In The Elasmobranch Fishes, pp. 268-295. Berkeley, CA: University of California Press. Døving, K. B., Hansson, K. A., Backstrom, T. and Hamdani, E. H. (2011). Visualizing a set of olfactory sensory neurons responding to a bile salt. J. Exp. Biol. 214, 80-87. Døving, K. B., Selset, R. and Thommesen, G. (1980). Olfactory Sensitivity to Bile-Acids in Salmonid Fishes. Acta Physiol. Scand. 108, 123-131. 94 Dryer, L. and Graziadei, P. P. C. (1993). A Pilot-Study on Morphological Compartmentalization and Heterogeneity in the Elasmobranch Olfactory-Bulb. Anat. Embryol. (Berl). 188, 4151. Eisthen, H. L. (2004). The goldfish knows: Olfactory receptor cell morphology predicts receptor gene expression. J. Comp. Neurol. 477, 341-346. Ferrando, S., Bottaro, M., Gallus, L., Girosi, L., Vacchi, M. and Tagliafierro, G. (2006). Observations of crypt neuron-like cells in the olfactory epithelium of a cartilaginous fish. Neurosci. Lett. 403, 280-282. Ferrando, S., Gambardella, C., Ravera, S., Bottero, S., Ferrando, T., Gallus, L., Manno, V., Salati, A. P., Ramoino, P. and Tagliafierro, G. (2009). Immunolocalization of G-Protein Alpha Subunits in the Olfactory System of the Cartilaginous Fish Scyliorhinus Canicula. Anatomical Record-Advances in Integrative Anatomy and Evolutionary Biology 292, 1771-1779. Firestein, S. (2001). How the olfactory system makes sense of scents. Nature 413, 218. Friedrich, R. W. and Korsching, S. I. (1997). Combinatorial and Chemotopic Odorant Coding in the Zebrafish Olfactory Bulb Visualized by Optical Imaging. Neuron 18, 737-752. Garman, S. (1913). The Plagiostomia (sharks, skates, and rays). Cambridge, MA: Printed for the museum. Hamdani, E. H. and Døving, K. B. (2007). The functional organization of the fish olfactory system. Prog. Neurobiol. 82, 80-86. Hansen, A., Anderson, K. T. and Finger, T. E. (2004). Differential distribution of olfactory receptor neurons in goldfish: Structural and molecular correlates. J. Comp. Neurol. 477, 347-359. Hansen, A. and Finger, T. E. (2000). Phyletic distribution of crypt-type olfactory receptor neurons in fishes. Brain. Behav. Evol. 55, 100-10. Hansen, A., Rolen, S. H., Anderson, K., Morita, Y., Caprio, J. and Finger, T. E. (2003). Correlation between Olfactory Receptor Cell Type and Function in the Channel Catfish. The Journal of Neuroscience 23, 9328-9339. 95 Hansen, A. and Zeiske, E. (1998). The peripheral olfactory organ of the zebrafish, Danio rerio: an ultrastructural study. Chem. Senses 23, 39-48. Hansen, A. and Zielinski, B. (2005). Diversity in the olfactory epithelium of bony fishes: Development, lamellar arrangement, sensory neuron cell types and transduction components. J. Neurocytol. 34, 183-208. Hara, T. J. (1994). The Diversity of Chemical Stimulation in Fish Olfaction and Gustation. Rev. Fish Biol. Fisher. 4, 1-35. Hildebrand, J. G. and Shepherd, G. M. (1997). Mechanisms of olfactory discrimination: Converging evidence for common principles across phyla. Annu. Rev. Neurosci. 20, 595631. Janvier, P. (1996). Early vertebrates. Oxford, England: Oxford University Press. Johnson, B. A. and Leon, M. (2007). Chemotopic odorant coding in a mammalian olfactory system. J. Comp. Neurol. 503, 1-34. Laberge, F. and Hara, T. J. (2001). Neurobiology of fish olfaction: a review. Brain Research Reviews 36, 46-59. Laframboise, A. J., Ren, X., Chang, S., Dubuc, R. and Zielinski, B. S. (2007). Olfactory sensory neurons in the sea lamprey display polymorphisms. Neurosci. Lett. 414, 277-281. Li, W., Scott, A. P., Siefkes, M. J., Yan, H., Liu, Q., Yun, S.-S. and Gage, D. A. (2002). Bile Acid Secreted by Male Sea Lamprey That Acts as a Sex Pheromone. Science 296, 138. Lipschitz, D. L. and Michel, W. C. (2002). Amino Acid Odorants Stimulate Microvillar Sensory Neurons. Chem. Senses 27, 277-286. Meredith, T. L. and Kajiura, S. M. (2010). Olfactory morphology and physiology of elasmobranchs. Journal of Experimental Biology 213, 3449-3456. Meredith, T. L., Kajiura, S. M. and Caprio, J. (In prep). Sensitivity and specificity of the elasmobranch olfactory epithelium to bile salts. Chem. Senses. 96 Ngai, J., Chess, A., Dowling, M. M., Necles, N., Macagno, E. R. and Axel, R. (1993). Coding of olfactory information: Topography of odorant receptor expression in the catfish olfactory epithelium. Cell 72, 680. Nikonov, A. A. and Caprio, J. (2001). Electrophysiological evidence for a chemotopy of biologically relevant odors in the olfactory bulb of the channel catfish. J. Neurophysiol. 86, 1869-1876. Nikonov, A. A. and Caprio, J. (2004). Odorant specificity of single olfactory bulb neurons to amino acids in the channel catfish. J. Neurophysiol. 92, 123-134. Norris, H. W. and Hughes, S. P. (1920). The cranial, occipital, and anterior spinal nerves of the dogfish, Squalus acanthias. The Journal of Comparative Neurology 31, 293-402. Northcutt, R. G. (1978). Brain organization in the cartilagenous fishes. In Sensory biology of shark, skates, and rays, eds. E. S. Hodgson and R. F. Mathewson), pp. 117-193. Washington, D. C.: Government Printing Press. Oka, Y., Ichikawa, M. and Ueda, K. (1982). Synaptic organization of the olfactory bulb and central projection of the olfactory tract. In Chemoreception in Fishes, (ed. T. J. Hara), pp. 61-76. Amsterdam: Elsevier. Reese, T. S. and Brightman, M. W. (1970). Olfactory surfcace and central olfactory connexions in some vertebrates. In Taste and smell in vertebrate, eds. G. E. W. Wolstenholme and J. Knight), pp. 115-149. London: J. & A. Churchill. Ressler, K. J., Sullivan, S. L. and Buck, L. B. (1993). A zonal organization of odorant receptor gene expression in the olfactory epithelium. Cell 73, 609. Riddle, D. and Oakley, B. (1991). Evaluation of projection patterns in the primary olfactory system of rainbow trout. J. Neurosci. 11, 3752-3762. Sato, K. and Suzuki, N. (2001). Whole-cell response characteristics of ciliated and microvillous olfactory receptor neurons to amino acids, pheromone candidates and urine in rainbow trout. Chem. Senses 26, 1145-1156. 97 Satou, M. (1992). Synaptic organization of the olfactory bulb and its central projection. In Fish Chemoreception, (ed. T. J. Hara), pp. 40-59. London: Chapman & Hall. Schluessel, V., Bennett, M. B., Bleckmann, H., Blomberg, S. and Collin, S. P. (2008). Morphometric and ultrastructural comparison of the olfactory system in elasmobranchs: The significance of structure-function relationships based on phylogeny and ecology. J. Morphol. 269, 1365-1386. Siefkes, M. J. and Li, W. (2004). Electrophysiological evidence for detection and discrimination of pheromonal bile acids by the olfactory epithelium of female sea lampreys (Petromyzon marinus). J. Comp. Physiol. A -Neuroethol. Sens. Neural Behav. Physiol. 190, 193-199. Silver, W. L. (1979). Olfactory Responses from a Marine Elasmobranch, the Atlantic Stingray, Dasyatis sabina. Mar. Behav. Physiol. 6, 297-305. Sorensen, P. W., Fine, J. M., Dvornikovs, V., Jeffrey, C. S., Shao, F., Wang, J. Z., Vrieze, L. A., Anderson, K. R. and Hoye, T. R. (2005). Mixture of new sulfated steroids functions as a migratory pheromone in the sea lamprey. Nat. Chem. Biol. 1, 324-328. Sorensen, P. W. and Stacey, N. E. (2004). Brief review of fish pheromones and discussion of their possible uses in the control of non-indigenous teleost fishes. N. Z. J. Mar. Freshw. Res. 38, 399-417. Strausfeld, N. J. and Hildebrand, J. G. (1999). Olfactory systems: common design, uncommon origins? Curr. Opin. Neurobiol. 9, 634-639. Takami, S., Luer, C. A. and Graziadei, P. C. (1994). Microscopic structre of the olfactory organ of the clearnose skate, Raja eglanteria. Anat. Embryol. (Berl). 190, 211-230. Tester, A. L. (1963). Olfaction, gustation and the common chemical sense in sharks. In Sharks and Survival, (ed. P. W. Gilbert), pp. 243-254. Boston, MA: DC Health. Theisen, B., Zeiske, E. and Breucker, H. (1986). Functional morphology of the olfactory organs in the spiny dogfish (Squalus acanthius L.) and the small-spotted catshark (Scyliorhinus canicula (L.)). Acta Zool. 67, 73-86. 98 Tricas, T. C., Kajiura, S. M. and Summers, A. P. (2009). Response of the hammerhead shark olfactory epithelium to amino acid stimuli. J. Comp. Physiol. A 195, 947-954. Vielma, A., Ardiles, A., Delgado, L. and Schmachtenberg, O. (2008). The elusive crypt olfactory receptor neuron: evidence for its stimulation by amino acids and cAMP pathway agonists. J. Exp. Biol. 211, 2417-2422. Yamamoto, M. (1982). Comparative morphology of the peripheral olfactory organ in teleosts. In Chemoreception in fishes, (ed. T. J. Hara), pp. 39-59. New York, NY: Elsevier. Zeiske, E., Caprio, J. and Gruber, S. H. (1986). Morphological and electrophysiological studies on the olfactory organ of the lemon shark, Negaprion brevirostris. In Indo-Pacific Fish Biology: Proceedings of the Second International Conference on Indo-Pacific Fishes, eds. T. Uyeno R. Arai T. Taniuchi and K. Matsuura), pp. 381-391. Tokyo: Ichtyological Society of Japan. Zeiske, E., Kasumyan, A., Bartsch, P. and Hansen, A. (2003). Early development of the olfactory organ in sturgeons of the genus Acipenser: a comparative and electron microscopic study. Anat. Embryol. (Berl). 206, 357-372. Zhang, C. B., Brown, S. B. and Hara, T. J. (2001). Biochemical and physiological evidence that bile acids produced and released by lake char (Salvelinus namaycush) function as chemical signals. J. Comp. Physiol. B-Biochem. Syst. Environ. Physiol. 171, 161-171. Zielinski, B. and Hara, T. J. (2006). Olfaction. In Fish Physiology: Sensory Systems Neuroscience, vol. 25 eds. B. Zielinski and T. J. Hara), pp. 1-43: Academic Press. 99 Figure 4.1 The three species used in this study represent two elasmobranch orders; Rajiformes (stingrays) and Carcharhiniformes (shark) (A). Photographs of the brains of the Atlantic stingray (D. sabina) and lemon shark (N. brevirostris) illustrate their differing OB morphologies. The OBs of D. sabina and D. say, which both occur as a cohesive unit, whereas the olfactory bulb (OB) of N. brevirostris occurs as two physically separate hemi-olfactory bulbs (B). A schematic diagram of the olfactory pathway in elasmobranchs (C) shows the olfactory organ that is comprised of several lamellae overlain with the secondarily folded olfactory epithelium (OE). Odorants that enter the olfactory capsule bind to molecular olfactory receptors (ORs) on the microvilli and cilia of the microvillous (blue) and crypt (green) olfactory receptor neurons (ORNs) in the OE. The ORN axons project through the lamina propria of each lamella, through the short olfactory nerve (not visible externally), and to the OB where they synapse with mitral cells at glomeruli. Line drawings of the Dasyatids are modified from McEachran and de Carvalho (2002), and the line drawing of the lemon shark is modified from Compagno (2002). The schematic in panel C is modified from Hamdani and Døving (2007) and Firestein (2001). 100 Figure 4.2 Morphology of the OB and OE of D. say visualized using KLO (nuclear red-light greenorange) staining. An inset in the top left corner illustrates the area of the olfactory bulb and organ being examined (gray shaded area). The axons of the ORNs situated in the folds of OE project through the lamina propria of each lamella and to the OB where they synapse with mitral cells at glomeruli. The laminar organization of the OB is apparent. A superficial connective tissue layer occurred at the posterior aspect of the OB. Deep to that layer, glomeruli (*) were distributed in a diffuse layer and arranged around the axons (dotted circle). 101 Figure 4.3 Retrograde labeling from the OB to the OE in both stingray species. An inset in the top left corner of each panel illustrates the dye type (green = Far Red and red = DiI), general dye placement location, and the area of the olfactory bulb and organ being examined (gray shaded area). Panels A and B show labeling of the glomeruli (*) and axons in the OB near the labeling site. DiI in the OB spread into the adjacent nerve fascicles and the axon bundles in the lamina propria of the three to five lamellae immediately anterior to the labeling site in the OB (Panel C). Labeling extended from those axon bundles into axons innervating the ORNs in the secondary folds of the OE (Panels D-F). Panel G shows a single microvillus ORN labeled with DiI. No labeling was seen in distant lamellae. 102 Figure 4.4 Retrograde labeling from the OB to the OE in the lemon shark, N. brevirostris. An inset in the top left corner of each panel illustrates the dye type (green = Far Red and red = DiI), general dye placement location, and the area of the olfactory bulb and organ being examined (gray shaded area). Panel A shows distinct labeling of the medial and lateral hemi-OBs. Panel B shows a DiI labeled medial hemi-OB, including axon bundles from the adjacent olfactory lamellae leading to several glomeruli (*). Panel C is a composite image of multiple olfactory lamellae labeled on the medial side with DiI and the lateral side with Far Red. No double labeling of lamellae was seen. Panels D and E show the olfactory epithelium and individual ORNs labeled with DiI. The sample in panel D was labeled with both dyes in the lateral hemi-OB; however, we saw no double labeling of the OE. 103 Figure 4.5 Retrograde labeling from the OB to the OE in lemon shark (N. brevirostris) samples that were labeled using both dyes in a single hemi-OB. An inset in the top left corner of each panel illustrates the dye type (green = Far Red and red = DiI), general dye placement location, and the area of the olfactory bulb and organ being examined (gray shaded area). Although the lateral hemi-OB was labeled with both dyes in the sample in Panel A, there was distinct labeling of axon bundles by each dye. In contrast, only when the two dyes were placed so closely that the same axons were affected we observed double labeling of axons in the OB, indicated by the yellow hue (Panel B). In samples where both dyes were placed in the same hemi-OB, we did not see double labeling of any lamellae or ORNs. 104 CHAPTER 5 GENERAL DISCUSSION ELASMOBRANCH OLFACTION RESEARCH Elasmobranch fishes are renowned for their remarkable sense of smell and are often referred to in the popular media as “swimming noses”. Unconfirmed assumptions about their olfactory sensitivities even persist in the current scientific literature. The majority of elasmobranch olfaction research focused on either chemo-orientation behavior or the morphology of the olfactory system, but significant gaps in our knowledge still remain. Behavioral research revealed at least three orientation methods (rheotaxis, klinotaxis, and tropotaxis) employed by different shark species to locate an odor source (Gardiner and Atema, 2010; Hodgson and Mathewson, 1971; Johnsen and Teeter, 1985). More recently, scientists are placing olfaction into a broader sensory context to determine how shark species integrate information from multiple sensory modalities to orient to food (Gardiner and Atema, 2007; Gardiner et al., 2011). For example, three shark species from different ecological niches were all demonstrated to use olfaction to track live prey from a distance, but in contrast to the other two species, the nurse shark (Ginglymostoma cirratum), a benthic, suction-feeder, completely stopped feeding when the olfactory nares were blocked (Gardiner et al., 2011). Although chemo-orientation behavior varies markedly among elasmobranch species, highlighting their ecological differences, these studies confirmed that olfaction is a critical component in food localization for elasmobranchs (Hodgson and Mathewson, 1971). Studies that describe the external olfactory anatomy often relate particular anatomical features to species‟ ecology or phylogeny, such as the placement of the nares or the presence of a naso-labial groove (Bell, 1993; Kajiura et al., 2005; Tester, 1963). Other studies examined the 105 gross morphology of the internal olfactory structures, usually the peripheral olfactory organ or olfactory bulb (OB) of the brain. This research often compared the volume or size of these structures in multiple species to infer aspects about their sensitivity and relative reliance on olfaction, though these assumptions were not directly tested (Lisney et al., 2007; Lisney and Collin, 2006; Schluessel et al., 2008). Also, few studies delved into the detailed organization of the neuronal projections to the OB and to higher order nuclei of the forebrain. Relatively recent advances in neuronal tracing techniques enabled the visualization of individual neural projections; however only two studies employed these techniques on the elasmobranch brain (Dryer and Graziadei, 1993; Dryer and Graziadei, 1994). One study, which examined the organization of olfactory receptor neuron (ORN) projections from the olfactory epithelium (OE) to the OB, suggested that elasmobranchs may possess a unique OB organization compared to teleost fishes and most other vertebrates (Dryer and Graziadei, 1993). Further studies are required to determine the extent of this organization of the OB of elasmobranchs and how it affects the processing of odor information. Morphological and behavioral studies on elasmobranch olfaction make many assumptions about the function of this sensory system; however, few studies investigated the olfactory physiology of elasmobranchs. For example, in a study of four wobbegong shark species (genus Orectolobus), the number of olfactory lamellae and the surface area of the OE were used to assess the relative olfactory sensitivity without actually quantifying olfactory thresholds (Theiss et al., 2009). Physiological experiments are essential in order to directly quantify the sensitivity and specificity of the elasmobranch olfactory system. Existing studies each used only single species and tested responses to a single odorant class, amino acids (Silver, 1979; Tricas et al., 2009; Zeiske et al., 1986). A multi-species study would more thoroughly address the olfactory capabilities of elasmobranchs and allow testing of assumptions about interspecific difference in olfactory capabilities as it relates to phylogeny or ecology. In addition, a wider diversity of odorant classes needs to be tested to help determine which odors are biologically relevant to elasmobranchs. 106 Compared to research on elasmobranch olfaction, researchers uncovered much more information on olfaction in teleost fishes and other more derived vertebrates, such as mammals. The genomes of the zebrafish, mouse, rat, and several other animals were sequenced, and the genes encoding for olfactory receptor (OR) protein expression have been identified. This allows for the use of molecular and genetic techniques not currently available in elasmobranch olfactory research. Due to this gap in knowledge, the OR gene expression in elasmobranchs is still unknown. Olfactory studies for these groups have moved far beyond simply determining olfactory sensitivity and specificity to odorants. For example, recent studies aimed to identify the genes involved in olfactory signal transduction in fish and compare them to those used in mammals (Oka and Korsching, 2011). Though olfaction research in elasmobranchs has fallen far behind that for other vertebrates, this group of fishes still holds the key to critical information about the evolution of this ancient sensory modality. Elasmobranchs are excellent models for the study of vertebrate olfaction based upon their basal location within the vertebrate clade, their diverse olfactory morphology, and their occupation of a wide variety of ecological niches. However, there is a general paucity of basic research on their olfactory capabilities, receptor types, and organization of olfactory input, which precludes comparisons with other vertebrate groups. In this dissertation, I examined the morphology, physiology, and organization of the olfactory system of representative elasmobranchs to facilitate comparisons of these cartilaginous fishes with the more derived teleost fishes and other vertebrates. RESULTS OF THE PRESENT STUDY Chapter 2: Olfactory morphology and physiology To test the hypotheses that elasmobranchs possess greater olfactory sensitivities than teleost fishes and that lamellar surface area is correlated to sensitivity, I compared the surface area of the olfactory lamellae (the location of the molecular ORs) and the olfactory sensitivities of five phylogenetically diverse elasmobranch species (the clearnose skate, Raja eglanteria Bosc 1800; yellow stingray, Urobatis jamaicensis (Cuvier 1816); Atlantic stingray, D. sabina (Lesueur 1824); lemon shark, N. brevirostris (Poey 1868); and bonnethead shark, Sphyrna tiburo 107 (Linnaeus 1758)). The lamellar surface area, not including the secondary epithelial folds, was calculated digitally for several individuals of each species using photographs of ten individually dissected lamellae from each olfactory organ. Using an electrophysiological technique, called an electro-olfactogram (EOG), I measured the responses of individual fish to twenty L-amino acid odorants at varying concentrations. Lamellar surface area varied interspecifically; however, it did not correlate with amino acid thresholds, which ranged from 10 –9 –6 to 10 M for all five species. Greater lamellar surface area may not confer greater olfactory sensitivity because it does not necessarily translate to a greater number or density of ORNs; also ORN quantity is only one of several factors that affect sensitivity. Other factors include the concentration of background odorants, the number of ORNs converging onto a specific glomerulus in the OB, and the binding affinities of ORNs for different odorants. Although elasmobranchs are reputed to possess a superior olfactory sensitivity, the amino acid thresholds reported in this study were comparable to those previously reported for the most prolific group of aquatic vertebrates, teleost fishes, and for aquatic invertebrates, such as crustaceans (10 –9 –7 to 10 M) (Caprio and Derby, 2008). This amino acid concentration range approximates the level of free amino acids in seawater (Hara, 1994; Kuznetsova et al., 2004; Pocklington, 1971). Since the olfactory threshold of an animal varies depending on the background level of odorants (Caprio, 1982) and many aquatic species are subject to similar environmental amino acid levels, they appear to have converged upon similar amino acid sensitivities. Chapter 3: Sensitivity and specificity to bile salts Bile salts, which are produced throughout the vertebrate clade, are biliary steroids created in the liver to facilitate intestinal absorption of lipids (Hagey et al., 2010; Haslewood, 1967; Hofmann et al., 2010). Bile salts are typically reabsorbed and reused by the enterohepatic system, but teleost fishes excrete a portion of their bile salts through their urine or feces which serve as potent olfactory stimuli, potentially as pheromones (Døving et al., 1980; Huertas et al., 2010; Velez et al., 2009; Zhang and Hara, 2009; Zhang et al., 2001). To test the hypothesis that 108 elasmobranchs are able to detect bile salt odorants despite lacking ciliated ORNs, the morphological type of ORN shown to mediate bile salt detection in the teleost olfactory system, I quantified the olfactory sensitivity and specificity of two elasmobranch species (the Atlantic stingray, D.sabina, and the bonnethead shark, S. tiburo) to four bile salts. This study demonstrated that elasmobranchs detect bile salt odorants despite lacking ciliated ORNs (Hansen et al., 2003; Hansen and Zielinski, 2005; Sato and Suzuki, 2001). The olfactory systems of both species responded to all four tested bile salt odorants, but demonstrated smaller relative EOG responses and less sensitivity compared to teleosts and agnathans (Huertas et al., 2010; Li et al., 1995; Michel and Derbidge, 1997; Michel and Lubomudrov, 1995; Siefkes and Li, 2004; Sorensen et al., 2005; Zhang and Hara, 2009). A potential reason for the difference in reported bile salt sensitivity between teleosts and elasmobranchs is that the bile salts we tested are produced by teleosts and are thought to be employed as pheromones (Sorensen and Caprio, 1997; Sorensen and Stacey, 2004; Zhang and Hara, 2009; Zhang et al., 2001). If elasmobranchs also use bile salts as pheromones, then bile salts that are produced by elasmobranchs, such as 5β-scymnol 27-sulfate, might prove more potent as olfactory stimuli, although they are not currently commercially available. Many vertebrates, including lampreys, teleost fishes, amphibians, reptiles, and mammals, possess ciliated ORNs utilizing the Gαolf transduction cascade (Eisthen, 2004), but elasmobranch fishes seem to lack both ciliated ORNs and Gαolf protein expression (Ferrando et al., 2009; Schluessel et al., 2008). Since teleost olfactory responses to bile salts are mediated by ciliated ORNs, which elasmobranchs lack, elasmobranchs must use either microvillous or crypt ORNs for bile salt detection, a strategy thus far undocumented in fishes. Elasmobranchs seem to detect a comparable suite of odorants as teleosts with fewer types of ORNs. In their investigation of the G–protein associations of morphologically different ORNs in teleosts, Hansen and Zielinski (2005) reported the presence of three sub-classes of microvillous ORNs, each coupled to a specific Gprotein α-subunit. It is possible that elasmobranchs possess multiple sub-types of microvillous and crypt ORNs with at least somewhat distinct odorant specificities. 109 Cross-adaptation experiments, which aim to determine whether two agonists interact with independent or overlapping OR populations, were also performed to test whether the receptor mechanism for bile salt sensitivity is independent from that for amino acids. The EOG responses of the two species were recorded to four test bile salts delivered separately over four amino acid adapting regimes. For both species, the responses to the bile salts during the adapted state did not differ significantly from those during the unadapted state. These results show that, like teleosts, the olfactory system of elasmobranchs contains molecular ORs for bile salts independent of those that detect amino acids (Michel and Derbidge, 1997; Zhang and Hara, 2009). In addition to being able to distinguish between amino acids and bile salts, teleosts and agnathans distinguish among bile salt odorants depending on the type and position of the conjugating group (non-, taurine-, or glycine-conjugated) (Li and Sorensen, 1997; Michel and Derbidge, 1997; Rolen and Caprio, 2007; Zhang and Hara, 2009). Further studies are needed to determine whether elasmobranchs discriminate among bile salts using the same mechanism. Chapter 4: Somatotopy of the hemi-olfactory bulb In many elasmobranch species, such as the Atlantic sharpnose shark (Rhizoprionadon terraenovae), each OB is physically partitioned into two or more “hemi-bulbs”. This hemi-bulb morphology is unique among verebrates and its functional significance is not fully understood (Northcutt, 1978; Tester, 1963). This study examined the organization of the OBs in three elasmobranch species (the bluntnose stingray, D. say, the Atlantic stingray, D. sabina, and the bonnethead shark, S. tiburo) with varying OB morphologies to test the hypothesis that axons projecting from the OE to the OB exhibit a somatotopic arrangement. Fluorescent, lipophilic dyes were inserted into the OB of each specimen to retrogradely label ORNs in the epithelium. The results indicate that the distribution of glomeruli in the OB is less defined when compared to certain teleost species. Also, glomeruli in the OB receive projections from ORNs in three to four olfactory lamellae situated immediately anteriorly, indicating a somatotopic organization of the elasmobranch OB. 110 Axonal tracing studies have shown that ORNs expressing a particular molecular OR type in the OE possess axons that converge to project to a localized region in the OB (Baier et al., 1994; Hansen et al., 2003; Hara and Zhang, 1997; Morita and Finger, 1998). In addition, electrophysiological studies have demonstrated that different OB regions respond preferentially to particular odorant classes (Døving et al., 2011; Friedrich and Korsching, 1997; Nikonov and Caprio, 2001). This confirms that the teleost OB is organized chemotopically, similar to virtually all other vertebrates researched to date (Hamdani and Døving, 2007). This chemotopic organization is thought to play an integral role in encoding odorant identity and concentration (Caprio and Derby, 2008; Hildebrand and Shepherd, 1997; Johnson and Leon, 2007; Nikonov and Caprio, 2001; Strausfeld and Hildebrand, 1999). The somatotopic organization found in the OBs of the elasmobranch species examined here has not been documented in any other vertebrate and could potentially be related to the hemi-OB morphology. FUTURE RESEARCH Understanding elasmobranchs Future olfaction research in elasmobranchs should continue to examine how the morphology and physiology of sensory systems influence species‟ ecology and behavior. Other odorant classes (e.g. polyamines, elasmobranch-produced bile salts, gonadal steroids, prostaglandins, and nucleotides) should be tested to determine the olfactory repertoire of elasmobranchs, and cross-adaptation and mixture experiments should be employed to discern the OR types present in the elasmobranch OE. The odorant specificities and detailed projections of the different ORN types need to be examined, not only to discriminate between microvillous and crypt ORNs, but also to identify potential subclasses of microvillous ORNs. This will also aid in understanding the unique OB organization. Many recent studies on agnathan and teleost olfaction focus on the use of bile salts, prostaglandins, and gonadal steroids as pheromones (chemical signals used by conspecifics), which mediate a variety of behaviors, such as migration and mating (Stacey and Sorensen, 2005). Only anecdotal evidence exists supporting the use of pheromones by elasmobranch 111 fishes. These include observations of a male shark apparently following the scent trail of a female shark (Johnson and Nelson, 1978), male sharks “nosing” a female‟s cloaca (Pratt and Carrier, 2001), and bite wounds concentrated on the posterior disc of female rays, all possibly due to the presence pheromones being released from the cloaca (Kajiura et al., 2000). Future research should aim to more conclusively demonstrate the use of pheromones by elasmobranchs. This would involve the identification of putative pheromones by analyzing the chemicals being released in the urine or feces of individuals, followed by electrophysiological experiments to determine whether these chemicals stimulate the olfactory system of conspecifics, and finally documentation of behavioral or endocrinological effects of those chemicals on conspecifics. Understanding olfaction Chemical sensitivity is present in nearly all life forms, from bacteria to mammals (Ache and Young, 2005; Firestein, 2001). The olfactory system is our most highly developed system for molecular sensing, and is able to sense and distinguish among thousands of compounds. Olfactory receptor genes make up the largest gene family (~1000 genes) in the mammalian genome; in rats they comprise ~6% of the genome, which is an extremely large proportion compared to other gene families. The olfactory system detects molecules using G-protein coupled receptors (GPCRs), which are biologically ubiquitous in eukaryotes. GPCRs are used to not only detect odors, but also light-sensitive compounds (such as rhodopsin) and neurotransmitters, and they are the target of an estimated 50% of drugs on the market or in development (Lagerström and Schioth, 2008). There is a remarkable degree of convergence in the organization of the olfactory pathways, from the use of GPCRs to the organization of the OB (or analogous structure), in a phylogenetically diverse array of animals (Ache and Young, 2005; Firestein, 2001). Animal models are useful not only in investigating this sense as it applies to higher vertebrates, but also in understanding the evolution of olfaction. As mentioned earlier, elasmobranchs are an excellent model for the study of olfaction based upon their basal location within the vertebrate clade, their 112 diverse olfactory morphology, and their occupation of a wide variety of ecological niches. This dissertation research aimed to add a new layer to our understanding of elasmobranch sensory biology and vertebrate olfaction. 113 REFERENCES Ache, B. W. and Young, J. M. (2005). Olfaction: Diverse Species, Conserved Principles. Neuron 48, 417-430. Baier, H., Rotter, S. and Korsching, S. (1994). Connectional Topography in the Zebrafish Olfactory System - Random Positions but Regular Spacing of Sensory Neurons Projecting to an Individual Glomerulus. Proc. Natl. Acad. Sci. U. S. A. 91, 11646-11650. Bell, M. A. (1993). Convergent evolution of nasal structure in sedentary elasmobranchs. Copeia 1, 144-158. Caprio, J. (1982). High sensitivity and specificity of olfactory and gustatory receptors of catfish to amino acids. In Chemoreception in Fishes, (ed. T. J. Hara), pp. 109-134. Amsterdam: Elsevier. Caprio, J. and Derby, C. D. (2008). Aquatic animal models in the study of chemoreception. In The Senses: A Comprehensive Reference, vol. 4: Olfaction and Taste eds. A. I. Basbaum A. Kaneko G. M. Shepherd and G. Westheimer), pp. 97-134. San Diego, CA: Academic Press. Døving, K. B., Hansson, K. A., Backstrom, T. and Hamdani, E. H. (2011). Visualizing a set of olfactory sensory neurons responding to a bile salt. J. Exp. Biol. 214, 80-87. Døving, K. B., Selset, R. and Thommesen, G. (1980). Olfactory Sensitivity to Bile-Acids in Salmonid Fishes. Acta Physiol. Scand. 108, 123-131. Dryer, L. and Graziadei, P. P. C. (1993). A Pilot-Study on Morphological Compartmentalization and Heterogeneity in the Elasmobranch Olfactory-Bulb. Anat. Embryol. (Berl). 188, 4151. Dryer, L. and Graziadei, P. P. C. (1994). Projections of the Olfactory Bulb in an Elasmobranch Fish, Sphyrna tiburo: Segregation of Inputs in the Telencephalon. Anat. Embryol. (Berl). 190, 563-572. Eisthen, H. L. (2004). The goldfish knows: Olfactory receptor cell morphology predicts receptor gene expression. J. Comp. Neurol. 477, 341-346. 114 Ferrando, S., Gambardella, C., Ravera, S., Bottero, S., Ferrando, T., Gallus, L., Manno, V., Salati, A. P., Ramoino, P. and Tagliafierro, G. (2009). Immunolocalization of G-Protein Alpha Subunits in the Olfactory System of the Cartilaginous Fish Scyliorhinus Canicula. Anatomical Record-Advances in Integrative Anatomy and Evolutionary Biology 292, 1771-1779. Firestein, S. (2001). How the olfactory system makes sense of scents. Nature 413, 218. Friedrich, R. W. and Korsching, S. I. (1997). Combinatorial and Chemotopic Odorant Coding in the Zebrafish Olfactory Bulb Visualized by Optical Imaging. Neuron 18, 737-752. Gardiner, J. M. and Atema, J. (2007). Sharks need the lateral line to locate odor sources: rheotaxis and eddy chemotaxis. J. Exp. Biol. 210, 1925-1934. Gardiner, J. M. and Atema, J. (2010). The Function of Bilateral Odor Arrival Time Differences in Olfactory Orientation of Sharks. Curr. Biol. 20, 1187-1191. Gardiner, J. M., Atema, J., Hueter, R. E. and Motta, P. J. (2011). Multisensory Integration in Shark Feeding Behavior. Integr. Comp. Biol. 51, E45-E45. Hagey, L. R., MØller, P. R., Hofmann, A. F. and Krasowski, M. D. (2010). Diversity of Bile Salts in Fish and Amphibians: Evolution of a Complex Biochemical Pathway. Physiol. Biochem. Zool. 83, 308-321. Hamdani, E. H. and Døving, K. B. (2007). The functional organization of the fish olfactory system. Prog. Neurobiol. 82, 80-86. Hansen, A., Rolen, S. H., Anderson, K., Morita, Y., Caprio, J. and Finger, T. E. (2003). Correlation between Olfactory Receptor Cell Type and Function in the Channel Catfish. The Journal of Neuroscience 23, 9328-9339. Hansen, A. and Zielinski, B. (2005). Diversity in the olfactory epithelium of bony fishes: Development, lamellar arrangement, sensory neuron cell types and transduction components. J. Neurocytol. 34, 183-208. Hara, T. J. (1994). The Diversity of Chemical Stimulation in Fish Olfaction and Gustation. Rev. Fish Biol. Fisher. 4, 1-35. 115 Hara, T. J. and Zhang, C. (1997). Topographic bulbar projections and dual neural pathways of the primary olfactory neurons in salmonid fishes. Neuroscience 82, 313. Haslewood, G. A. D. (1967). Bile salt evolution. J. Lipid Res. 8, 535-550. Hildebrand, J. G. and Shepherd, G. M. (1997). Mechanisms of olfactory discrimination: Converging evidence for common principles across phyla. Annu. Rev. Neurosci. 20, 595631. Hodgson, E. S. and Mathewson, R. F. (1971). Chemosensory orientation in sharks. Annals of the New York Academy of Science, 175-181. Hofmann, A. F., Hagey, L. R. and Krasowski, M. D. (2010). Bile salts of vertebrates: structural variation and possible evolutionary significance. J. Lipid Res. 51, 226-246. Huertas, M., Hagey, L., Hofmann, A. F., Cerda, J., Canario, A. V. M. and Hubbard, P. C. (2010). Olfactory sensitivity to bile fluid and bile salts in the European eel (Anguilla anguilla), goldfish (Carassius auratus) and Mozambique tilapia (Oreochromis mossambicus) suggests a 'broad range' sensitivity not confined to those produced by conspecifics alone. J. Exp. Biol. 213, 308-317. Johnsen, P. B. and Teeter, J. H. (1985). Behavioral responses of bonnethead sharks (Sphyrna) to controlled olfactory stimulation. Marine Behavior and Physiology 11, 283-291. Johnson, B. A. and Leon, M. (2007). Chemotopic odorant coding in a mammalian olfactory system. J. Comp. Neurol. 503, 1-34. Johnson, R. H. and Nelson, D. R. (1978). Copulation and possible olfaction-mediated pair formation in two species of carcharhinid sharks. Copeia 3, 539-542. Kajiura, S. M., Forni, J. B. and Summers, A. P. (2005). Olfactory morphology of carcharhinid and sphyrnid sharks: Does the cephalofoil confer a sensory advantage? J. Morphol. 264, 253263. Kajiura, S. M., Sebastian, A. P. and Tricas, T. C. (2000). Dermal Bite Wounds as Indicators of Reproductive Seasonality and Behaviour in the Atlantic Stingray, Dasyatis sabina. Environ. Biol. Fishes 58, 23-31. 116 Kuznetsova, M., Lee, C., Aller, J. and Frew, N. (2004). Enrichment of Amino Acids in the Sea Surface Microlayer at Coastal and Open Ocean Sites in the North Atlantic Ocean. Limnol Oceanogr 49, 1605-1619. Lagerström, M. C. and Schioth, H. B. (2008). Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat. Rev. Drug Discov. 7, 339-357. Li, W., Sorensen, P. and Gallaher, D. (1995). The olfactory system of migratory adult sea lamprey (Petromyzon marinus) is specifically and acutely sensitive to unique bile acids released by conspecific larvae. J. Gen. Physiol. 105, 569-587. Li, W. and Sorensen, P. W. (1997). Highly independent olfactory receptor sites for naturally occurring bile acids in the sea lamprey, Petromyzon marinus. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology 180, 429-438. Lisney, T. J., Bennett, M. B. and Collin, S. P. (2007). Volumetric shift of sensory brain areas indicates ontogenetic shifts in the relative importance of sensory systems in elasmobranchs. The Raffles Bulletin of Zoology Supplement No. 14, 7-15. Lisney, T. J. and Collin, S. P. (2006). Brain morphology in large pelagic fishes: a comparison between sharks and teleosts. J Fish Biol 68, 532-554. Michel, W. C. and Derbidge, D. S. (1997). Evidence of distinct amino acid and bile salt receptors in the olfactory system of the zebrafish, Danio rerio. Brain Res. 764, 179-187. Michel, W. C. and Lubomudrov, L. M. (1995). Specificity and Sensitivity of the Olfactory Organ of the Zebrafish, Danio rerio. J. Comp. Physiol. A-Sens. Neural Behav. Physiol. 177, 191199. Morita, Y. and Finger, T. E. (1998). Differential projections of ciliated and microvillous olfactory receptor cells in the catfish, Ictalurus punctatus. The Journal of Comparative Neurology 398, 539-550. Nikonov, A. A. and Caprio, J. (2001). Electrophysiological evidence for a chemotopy of biologically relevant odors in the olfactory bulb of the channel catfish. J. Neurophysiol. 86, 1869-1876. 117 Northcutt, R. G. (1978). Brain organization in the cartilagenous fishes. In Sensory biology of shark, skates, and rays, eds. E. S. Hodgson and R. F. Mathewson), pp. 117-193. Washington, D. C.: Government Printing Press. Oka, Y. and Korsching, S. I. (2011). Shared and Unique G Alpha Proteins in the Zebrafish Versus Mammalian Senses of Taste and Smell. Chem. Senses 36, 357-365. Pocklington, R. (1971). Physical Sciences: Free Amino-acids dissolved in North Atlantic Ocean Waters. 230, 374-375. Pratt, H. L. and Carrier, J. C. (2001). A Review of Elasmobranch Reproductive Behavior with a Case Study on the Nurse Shark, Ginglymostoma cirratum. Environ. Biol. Fishes 60, 188. Rolen, S. H. and Caprio, J. (2007). Processing of bile salt odor information by single olfactory bulb neurons in the channel catfish. J. Neurophysiol. 97, 4058-4068. Sato, K. and Suzuki, N. (2001). Whole-cell response characteristics of ciliated and microvillous olfactory receptor neurons to amino acids, pheromone candidates and urine in rainbow trout. Chem. Senses 26, 1145-1156. Schluessel, V., Bennett, M. B., Bleckmann, H., Blomberg, S. and Collin, S. P. (2008). Morphometric and ultrastructural comparison of the olfactory system in elasmobranchs: The significance of structure-function relationships based on phylogeny and ecology. J. Morphol. 269, 1365-1386. Siefkes, M. J. and Li, W. (2004). Electrophysiological evidence for detection and discrimination of pheromonal bile acids by the olfactory epithelium of female sea lampreys (Petromyzon marinus). J. Comp. Physiol. A -Neuroethol. Sens. Neural Behav. Physiol. 190, 193-199. Silver, W. L. (1979). Olfactory Responses from a Marine Elasmobranch, the Atlantic Stingray, Dasyatis sabina. Mar. Behav. Physiol. 6, 297-305. Sorensen, P. W. and Caprio, J. (1997). Chemoreception. In The Physiology of Fishes, (ed. D. H. Evans), pp. 375-405. Boca Raton, FL: CRC Press. 118 Sorensen, P. W., Fine, J. M., Dvornikovs, V., Jeffrey, C. S., Shao, F., Wang, J. Z., Vrieze, L. A., Anderson, K. R. and Hoye, T. R. (2005). Mixture of new sulfated steroids functions as a migratory pheromone in the sea lamprey. Nat. Chem. Biol. 1, 324-328. Sorensen, P. W. and Stacey, N. E. (2004). Brief review of fish pheromones and discussion of their possible uses in the control of non-indigenous teleost fishes. N. Z. J. Mar. Freshw. Res. 38, 399-417. Stacey, N. E. and Sorensen, P. W. (2005). Hormones, pheromones, and reproductive behaviors. In Behaviour: Interactions with Fish Physiology, vol. 24 eds. K. A. Sloman S. Balshine and R. W. Wilson), pp. 359-412. New York, NY: Academic Press. Strausfeld, N. J. and Hildebrand, J. G. (1999). Olfactory systems: common design, uncommon origins? Curr. Opin. Neurobiol. 9, 634-639. Tester, A. L. (1963). Olfaction, gustation and the common chemical sense in sharks. In Sharks and Survival, (ed. P. W. Gilbert), pp. 243-254. Boston, MA: DC Health. Theiss, S. M., Hart, N. S. and Collin, S. P. (2009). Morphological Indicators of Olfactory Capability in Wobbegong Sharks (Orectolobidae, Elasmobranchii). Brain Behav. Evolut. 73, 91-101. Tricas, T. C., Kajiura, S. M. and Summers, A. P. (2009). Response of the hammerhead shark olfactory epithelium to amino acid stimuli. J. Comp. Physiol. A 195, 947-954. Velez, Z., Hubbard, P. C., Welham, K., Hardege, J. D., Barata, E. N. and Canario, A. V. M. (2009). Identification, release and olfactory detection of bile salts in the intestinal fluid of the Senegalese sole (Solea senegalensis). J. Comp. Physiol. A -Neuroethol. Sens. Neural Behav. Physiol. 195, 691-698. Zeiske, E., Caprio, J. and Gruber, S. H. (1986). Morphological and electrophysiological studies on the olfactory organ of the lemon shark, Negaprion brevirostris. In Indo-Pacific Fish Biology: Proceedings of the Second International Conference on Indo-Pacific Fishes, eds. T. Uyeno R. Arai T. Taniuchi and K. Matsuura), pp. 381-391. Tokyo: Ichtyological Society of Japan. 119 Zhang, C. and Hara, T. J. (2009). Lake char (Salvelinus namaycush) olfactory neurons are highly sensitive and specific to bile acids. J. Comp. Physiol. A -Neuroethol. Sens. Neural Behav. Physiol. 195, 203-215. Zhang, C. B., Brown, S. B. and Hara, T. J. (2001). Biochemical and physiological evidence that bile acids produced and released by lake char (Salvelinus namaycush) function as chemical signals. J. Comp. Physiol. B-Biochem. Syst. Environ. Physiol. 171, 161-171. 120