Lab #1 Blackworm
advertisement

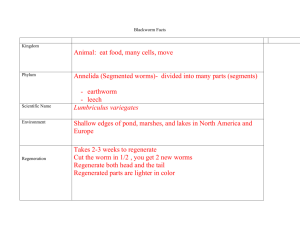
Biology/Hennings Lab #1 Biology 1-­‐2 1 Lab #1: Lumbriculus Contraction Rate of the Dorsal Blood Vessel Reading and Annotating Instructions for Handouts/Labs: Read the document SLOWLY. Underline and define any terms you do not know. If you are unfamiliar with a type of material or procedure look it up online. Background Reading: This amazing worm, whose name is Lumbriculus can be cut into several fragments—and it won’t die or even bleed. Instead, it regenerates a new head or tail, or both, from the various pieces. What’s more amazing is that the blackworm is not a rare animal living in some faraway place. Usually no more than 10 centimeters long, this worm lives in the shallow edges of ponds, marshes, and lakes throughout North America and Europe. Despite its short length, a mature blackworm has between 150 and 250 body segments. Even a fragment of blackworm that is only a few segments long can regenerate lost body parts—fast. In fact, fragmentation, followed by regeneration, is much more common than sexual reproduction in black worms. “The segments regenerate quickly,” says Dr. Charles Drewes, a zoologist who has studied black worms for many years. “For example, a new head or tail usually develops within 2 to 3 weeks. The new segments—usually eight for a head and between 20 and 100 for a tail—are smaller and paler than the original ones.” The blackworm “swims” by twisting its body through the water in a corkscrew fashion. If the water in which it lives is shallow enough, a black worm will stretch its tail to the surface of the water. It then bends its tail at a right angle so that a few centimeters of its dorsal surface is lying just above the water’s surface. Part of the tail now faces skyward and is exposed to air. Although this is a good position for gas exchange of oxygen and carbon dioxide, it exposes the blackworm’s tail to its enemies. To offset the problem of the tail’s exposure, the worm uses a special rapid escape reflex. The tail end rapidly shortens in response to a threatening enemy. This reflex can be triggered by touch, a vibration, or even by the sudden appearance of a shadow. Nerve cells, called “photoreceptors,” which are able to detect these shadows, are located in the blackworm’s tail. If you haven’t seen a blackworm in the wild, you’ve likely seen its relative, Lumbricus terrestris, the common earthworm. It, too, lives throughout North America and Europe—but in the soil. It can grow up to 25 centimeters long, and like the blackworm, it has the gift of regeneration. A mature earthworm has about 150 segments. It also has a light-colored bulge on its body, called the clitellum. If an earthworm is cut in two, only the part with the clitellum can regenerate. The part without the clitellum will die. Biology/Hennings Lab #1 Biology 1-­‐2 2 The next time you see an earthworm, look for its clitellum. Look even more carefully and you’ll also see tiny hairs on each segment of its body. These hairs, called setae (SEE-tee), help earthworms move by giving them many tiny grips on the soil. In blackworms, similar hairs are referred to as chaetae. Earthworms have remarkable regeneration powers, and they are also terrific diggers. These minibulldozers actually plow and fertilize soil! Here’s how: First, they eat bits of soil, decaying leaves, and bacteria and other microorganisms. (Each bite enlarges their network of underground tunnels.) With digestive systems the length of their bodies, they next grind and mix their food. Then they expel their waste, called castings, which is actually first-rate, nutrient-rich soil. Throughout this process, these tiny farmers till the earth by bringing sub- soil to the surface. That’s not all! Their tunnels give air and water easy access to the roots of plants, helping them to grow. Blackworms have a closed circulatory system with bright red blood that looks similar to human blood. In human blood there is a protein called hemoglobin that carries oxygen and carbon dioxide. Blackworms do not have hemoglobin. Instead they have a respiratory pigment called erythrocruorin that is dissolved in the plasma of their blood. There are two major blood vessels which are called the dorsal and ventral blood vessels. There are also lateral vessels that connect the dorsal and ventral vessels in anterior segments. Blood is moved by the waves of muscle constriction that begin near the posterior end of the dorsal vessel. The blackworms used in this lab will have been sitting in clean spring water for the past 24 hours which has allowed their digestive tract to empty. Objectives: You will utilize the scientific method to determine the contract rate of the dorsal blood vessel in the blackworm Lumbriculus. You will then design an experiment, collect and analyze data regarding how the contract rate of the blood vessel changes in response to a particular environmental stimulus and make a formal research poster. Safety Precautions: Materials: 1. Lumbriculus 1 for part I and if needed a second for part II 2. Dropping pipets (2) 3. Paper Towel- small handful 4. Stopwatch (1) 5. Microscope Slides with Parafilm Viewing Chamber (2) 6. Spring water 30 mL total per group 7. Glass Beaker 50 mL to hold the spring water (1) 8. Steromicroscope or compound light microscope (40x magnification) 9. Capillary Tubes (2) ** Optional 10. **For part II you will need other materials depending on your experimental design Biology/Hennings Lab #1 Biology 1-­‐2 3 Lab Partner: Name __________________________ Contact Info: __________________ Name __________________________ Contact Info: __________________ Part I Directions: 1. Go to your assigned lab station with your assigned lab partner. Exchange names and contact information (this could be cell #, or other social media info). Write this information above. 2. Put on safety goggles. 3. Gather all materials at your lab station. 4. Take one special microscope slide that has been prepared as a parafilm viewing chamber. In the middle of this special slide is a trough for the worm to be placed in. This is to help contain the blackworm and water to the middle of the slide. Look at the diagram below. Place the worm in the indented trough in the middle of the slide. 5. Go to the worm container at the front of the room and pick up one worm with the new dropping pipet. Submerge the pipet into the water that contains the worms and squeeze the bulb tip until you have a worm into the pipet. Take this back to your lab station carefully. Place the worm on the microscope slide inside the trough. Make sure the worm is submerged in water. Be careful to put enough water to fill in the trough but not too much to overflow outside of this chamber. If you have too much water use a paper towel to absorb the excess. 6. Place the slide on microscope stage or viewing area. Use the focus knobs on the microscope and bring the specimen clearly into view. You should have reviewed how to use both a compound light microscope and dissecting microscope before lab using the videos provided. If you need a quick refresher- please ask Mrs. Hennings. Identify the anterior and posteriors ends of the worm by using the diagram below. Each contraction begins as the posterior end of the worm travels forward. Biology/Hennings Lab #1 Biology 1-­‐2 4 Anatomy Vocab Check?? CAN YOU DEFINE? 1. Anterior 2. Posterior 3. Dorsal 4. Ventral 7. One lab partner should be the timer and one partner should be the recorder. 8. Choose a location in the posterior one-third of the worm in which to count contractions. Begin your count at 0 when the vessel is relaxed at that location, and then count the number of contractions that occur at that point for 30 seconds. Repeat for six counts and then average, rounding to the nearest whole. Record the data. 9. Repeat this procedure for a point in the middle one-third of the worm and again for a point in the anterior one- third of the worm. Record the data in table #1. Table #1 Biology/Hennings Lab #1 Biology 1-­‐2 5 Post Lab Questions Part I: Is the contraction rate the same for all three regions? Explain. _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ List some of the things that you think might influence the rate of contraction and explain each. _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ __________________________________________________________________________________ Part II Additional Available Materials • Nicotine powder (No-Doze) • Caffeine powder • Ice • Warm Water • Capsaicin (red pepper) • Ethanol • Ginseng Extract • d- limonene (orange peel fluid) You will now come up with an experimental hypothesis related to how one of the substances that are available would influence the rate of the contractions in the posterior section only. Write the experimental hypothesis in the “if…then….” Format. Experimental Hypothesis: __________________________________________________________________________________ __________________________________________________________________________________ Biology/Hennings Lab #1 Biology 1-­‐2 6 Now- you will write a “Null Hypothesis”. This is what you want to reject. You should phrase the null that there will not be any statistically significant difference between the means of the experimental and control groups with respect to …... Null Hypothesis: __________________________________________________________________________________ __________________________________________________________________________________ What is the independent variable (manipulated variable)? Be specific and include units! __________________________________________________________________________________ __________________________________________________________________________________ What is the dependent variable (responding variable)? Be specific and include units! __________________________________________________________________________________ __________________________________________________________________________________ What is the control group? Why is it necessary to have a control group? __________________________________________________________________________________ __________________________________________________________________________________ __________________________________________________________________________________ What are the constants that will be kept the same between the experimental and control groups? (Hint… there should be many!) __________________________________________________________________________________ __________________________________________________________________________________ __________________________________________________________________________________ __________________________________________________________________________________ Biology/Hennings Lab #1 Biology 1-­‐2 7 Make the experimental variable solution. You can choose to make either a 1:10 or a 1:100 solution. You will want to make enough solution for 6 trials. Put your solution in a labeled bottle. You will need to measure using the metric system and be VERY CAREFUL about recording accurate measurements. You may design your own procedure however- you will need to keep the worms in the solution for 15 minutes before checking the effects and then return them to pure spring water after exposure. Design a data table to write down your data for all 6 trials. Take pictures using a phone as you are doing experiment both of your researchers and of the experiment. __________________________________________________________________________________ Lab Poster Directions 1. Take a large sheet of poster paper and write both of your names and period of biology in the upper left corner. 2. Include a title for your inquiry lab that includes the dependent and independent variables including units. 3. List the following: a. Experimental Hypothesis b. Null Hypothesis c. Independent variable d. Dependent variable e. Control group f. Constants 4. Procedure: Flow Chart that shows exactly what was done. Include at least 3 photographs taken and printed. Two of the photographs should include images of the researchers. One photograph should be taken through the microscope and point out a distinguishing feature of the experiment. Include all relevant calculations made as far as making solutions. 5. Data Table that neatly and clearly shows the data from all 6 trials with proper headings. 6. Graph using the large graph paper- do this in pencil first and then go over it in sharpie. Graph the average of the 6 trials for both the control group and experimental group as a bar graph. Include standard error bars on the graph. GRAPHS MUST FOLLOW RULES OF GRAPHING! 7. Graph the exact data for all 6 trials on another piece of large graph paper with two different colored lines- one for the experimental group and another for the control group. Include properly labeled standard error bars on the graph. Biology/Hennings Lab #1 Biology 1-­‐2 8 8. Calculations section: a. Include how the mean was calculated b. Include how SD and SE were calculated 9. Analysis: Include an analysis of 10 sentences that uses quantitative data to reject or fail to reject the null hypothesis. Include at least 2 outside reliable sources that you will reference in APA format. You must clearly address how the independent variable affected the blood flow rate. 10. Conclusion: Include a conclusion using 3 sentences. 11. Works Cited: Include a works cited typed list with 2 reliable sources in APA format done on Easy Bib and printed.