Liver
advertisement

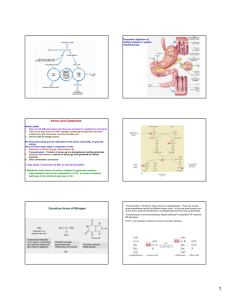
AMONYAK DETOXİFİKASYONU Doç Dr Osman EVLİYAOĞLU Ammonia is a toxic substance to plants and animals (especially for brain) Normal concentration: 25-40 mol/l (0.4-0.7 mg/l) Ammonia must be removed from the organism Peripheral Tissues Transport Nitrogen to the Liver Two ways of nitrogen transport from peripheral tissues (muscle) to the liver: Glutamate is not deaminated 1. Alanine cycle. Glutamate is formed by transamination reactions in peripheral tissues Nitrogen is then transferred to pyruvate to form alanine, which is released into the blood. The liver takes up the alanine and converts it back into pyruvate by transamination. The glutamate formed in the liver is deaminated and ammonia is utilized in urea cycle. 2. Nitrogen can be transported as glutamine. Glutamine synthetase catalyzes the synthesis of glutamine from glutamate and NH4+ in an ATPdependent reaction: Overview of Amino Acid Catabolism: Interorgan Relationships Overview of Amino Acid Catabolism: Interorgan Relationships Intestine Dietary amino acids absorbed Utilizes glutamine and asparagine as energy sources Releases CO2, ammonium, alanine, citrulline as endproducts Utilizes glutamine during fasting for energy Dietary amino acids and catabolites released to portal blood Overview of Amino Acid Catabolism: Interorgan Relationships Liver Synthesis of liver and plasma proteins Catabolism of amino acids Gluconeogenesis Ketogenesis Branched chain amino acids not catabolized Urea synthesis Amino acids released into general circulation Enriched (% of total aa) in BCAA (2-3X) Overview of Amino Acid Catabolism: Interorgan Relationships Skeletal Muscle Muscle protein synthesis Catabolism of BCAA Amino groups transported away as alanine and glutamine (50% of AA released) Alanine to liver for gluconeogenesis Glutamine to kidneys Kidney Glutamine metabolized to a-KG + NH4 a-KG for gluconeogenesis NH4 excreted or used for urea cycle (arginine synthesis) Important buffer preventing acidosis [NH4+] : [NH3] = 100 : 1 Overview of Amino Acid Catabolism: Interorgan Relationships Vitamin-Coenzymes in Amino Acid Metabolism Vitamin B-6 (pyridoxal phosphate) Folic acid (tetrahydrofolate) Vitamin B-12 Vitamin-Coenzymes in Amino Acid Metabolism Vitamin B-6 : pyridoxal phosphate Enzymes that bind amino acids use PLP as coenzyme for binding Transaminases Amino acid decarboxylases Amino acid deaminases Vitamin-Coenzymes in Amino Acid Metabolism Folacin: Tetrahydrofolate (THF) Carrier of single carbons Donor & receptor Glycine and serine Tryptophan degradation Histidine degradation Purine and pyrimidine synthesis Vitamin-Coenzymes in Amino Acid Metabolism Vitamin B-12 Catabolism of BCAA Methyl-malonyl CoA mutase (25-9 &10) Vitamin-Coenzymes in Amino Acid Metabolism Vitamin B-12 Methionine synthesis/recycling Methionine as a methyl donor Choline and creatine synthesis Homocysteine is product HCys -> Met requires B12 Figure 26-4 Disposal of Amino Acids Nitrogen: Key reactions Transamination reactions Deamination reactions Glutamate dehydrogenase Hydrolytic deamination Glutaminase Glutamine synthesis Disposal of Amino Groups: Transamination Reactions Transaminase reactions are reversible ALT = SGOT ALA important in muscle where ~25% of AA-N is transported out on ALA In liver, reverse reaction moves AA-N back on GLU AST = SGPT ASP important in liver since half of urea-N is from ASP Disposal of Amino Groups: Deamination Reactions Glutamate dehydrogenase oxidative deamination Important in liver where it releases ammonia for urea synthesis Hydrolytic deamination Glutaminase & asparaginase Disposal of Amino Groups: Glutamine Synthetase Important plasma transport form of nitrogen from muscle Detoxification of ammonia Brain Liver Removes ammonia intestinal tract Bacterial deamination of amino acids Glutamine utilization in intestinal cells Overview of Amino Acid Catabolism: Interorgan Relationships Detoxification of Ammonia by the Liver: the Urea Cycle Contains all enzyme of urea cycle Site of urea synthesis Kidney has all urea cycle enzymes except arginase Site of arginine synthesis Mitochondria CPS regulatory enzyme Flow of Nitrogen from Amino Acids to Urea in Liver Amino acid flow from muscle to liver Alanine & glutamine Liver Transfers N to GLU GLN’ase & GDH Transaminases Transfers GLU-N to: ASP AST Transamination route NH3 GDH Trans-deamination route GLN’ase Transfers N to urea Ammonia detoxification by the liver Liver very effective at eliminating ammonia from blood Periportal hepatocytes Portal blood ammonia = 300 – 1000 uM Systemic blood ammonia = 20uM Urea synthesis Km CPS ~ 1mM Perivenous hepatocytes Glutamine synthesis Very low Km for ammonia Removes any NH3 not removed by periportal hepatocytes THE UREA CYCLE Urea cycle - a cyclic pathway of urea synthesis first postulated by H.Krebs The sources of nitrogen atoms in urea molecule: - aspartate; - NH4+. Carbon atom comes from CO2. The free ammonia is coupling with carbon dioxide to form carbamoyl phosphate Two molecules of ATP are required Reaction takes place in the matrix of liver mitochondria Enzyme: carbamoyl phosphate synthetase (20 % of the protein of mitochondrial matrix) Carbamoyl ornithine phosphate donates carbamoyl group to The product - citruilline Enzyme: ornithine carbamoyltransferase Reaction takes place in the mitochondrial matrix Citrulline leaves the matrix and passes to the cytosol In the cytosol citrulline in the presence of ATP reacts with aspartate to form argininosuccinate Enzyme: argininosuccinate synthetase Argininosuccinate is cleaved to free arginine and fumarate Enzyme: argininosuccinate lyase The fumarate enters the tricarboxylic acid cycle Arginine is hydrolyzed to generate urea and ornithine Enzyme: arginase (present only in liver of ureotelic animals) Ornithine is transported back into the mitochondrion to begin another cycle Urea is excreted (about 40 g per day)