Chemical approach for interconversion of (S)
advertisement

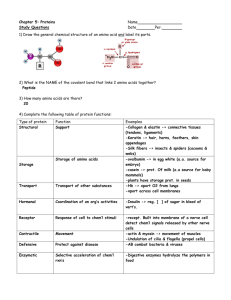
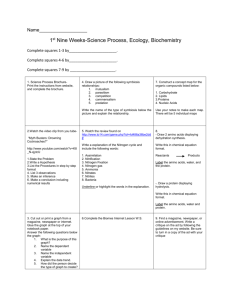
Organic & Biomolecular Chemistry View Article Online PAPER Published on 13 May 2013. Downloaded on 18/02/2014 13:49:00. Cite this: Org. Biomol. Chem., 2013, 11, 4503 Received 18th March 2013, Accepted 12th May 2013 View Journal | View Issue Chemical approach for interconversion of (S)- and (R)-α-amino acids Alexander E. Sorochinsky,a,b,c Hisanori Ueki,d José Luis Aceña,a Trevor K. Ellis,e Hiroki Moriwaki,f Tatsunori Satof and Vadim A. Soloshonok*a,b Here we report a general method for the preparation of unnatural (R)-α-amino acids via complexation of α-( phenyl)ethylamine derived chiral reagent (S)-3 with various (S)-α-amino acids. The reactions proceed DOI: 10.1039/c3ob40541a with synthetically useful chemical yields and thermodynamically controlled diastereoselectivity. Chiral reagent (S)-3 can be conveniently recovered and reused without any loss of enantiomeric purity and www.rsc.org/obc reactivity. Introduction D-α-Amino acids are rare in nature as compared with the corresponding L-enantiomers. Being “foreign” to the chemistry of life, naturally occurring D-α-amino acids have an important role in defense mechanisms (cell wall, venoms) of many microorganisms and plants. D-Amino acids have also been detected in a variety of peptides synthesized by animal cells, and several enzymes producing or metabolizing D-amino acids have been discovered.1 In recent decades, D-α-amino acids have been increasingly important in the development of new fields of biochemistry, in particular, enzymology, drug discovery and immune responses. However, the key driving factor for growth in the D-amino acids market is their applications in the pharmaceutical industry for the manufacture of pharmaceutical drugs and intermediates.2 Currently, commercially available D-α-amino acids are produced by biocatalytic methods, which are economically attractive, but have certain substrate limitations and rather low chemical efficiency.3 On the other hand, synthetic approaches, such as asymmetric synthesis and resolving reagents, can enjoy a potentially greater structural generality and overall efficiency, providing a cost structure comparable to biocatalytic methods. a Department of Organic Chemistry I, Faculty of Chemistry, University of the Basque Country UPV/EHU, 20018 San Sebastián, Spain. E-mail: vadym.soloshonok@ehu.es; Fax: +34 943-015270; Tel: +34 943-015177 b IKERBASQUE, Basque Foundation for Science, 48011 Bilbao, Spain c Institute of Bioorganic Chemistry & Petrochemistry, National Academy of Sciences of Ukraine, Murmanska 1, Kyiv 02660, Ukraine d International Center for Materials Nanoarchitectonics (MANA), National Institute for Materials Science (NIMS), 1-1, Namiki, Tsukuba, Ibaraki 305-0044, Japan e Department of Chemistry and Physics, 100 Campus Dr., Weatherford, OK 73096-3098, USA f Hamari Chemicals Ltd, 1-4-29 Kunijima, Higashi-Yodogawa-ku, Osaka 533-0024, Japan This journal is © The Royal Society of Chemistry 2013 Fig. 1 Chiral reagents 1–3 for the resolution/deracemization of α-amino acids. Taking into account that many L-amino acids are available from inexpensive and renewable natural sources, the development of chemical methods for the conversion of L-α-amino acids to the corresponding D-enantiomers seems to be of great synthetic potential. Recently, the Solladié-Cavallo4 group as well as the groups of Kim and Chin5 reported compounds 1 and 2 (Fig. 1), respectively, as useful new reagents for resolution/deracemization or interconversion of (R) and (S)-α-amino acids. In the case of compound (R)-1, the chiral amino acid forms a cyclic imino-ester allowing for reasonably good α-(R) stereocontrol (dr ∼ 90/10) by the quaternary stereogenic center. By contrast, reagent 2 does not form cyclic intermediates. Org. Biomol. Chem., 2013, 11, 4503–4507 | 4503 View Article Online Published on 13 May 2013. Downloaded on 18/02/2014 13:49:00. Paper Organic & Biomolecular Chemistry However, the intricate network of hydrogen bonding, involving the urea moiety and the hydroxyl group, provides for a high level (dr ∼ 95/5) of asymmetric induction in controlling the stereochemistry of the amino acid residue. The advantages of the design of reagents 1 and 2 are that they are recyclable, nonracemizable, available in both enantiomeric forms and react with unprotected amino acids under mild conditions. As for disadvantages, one can mention their high cost and incomplete diastereoselectivity. Recently, our group has designed reagent 3 and demonstrated its application to the deracemization of α-amino acids.6 While deracemization and interconversion of (R)- and (S)α-amino acids involve similar reaction chemistry (enolization followed by diastereoselective protonation), one might agree that this type of new application of reagent 3 should be separately studied and supported by experimental data. Here we report our results on the interconversion of (R)- and (S)α-amino acids using both enantiomeric forms of reagent 3. We also disclose additional modifications of the reaction conditions allowing for an improvement of the reactivity and performance of reagent 3. Results and discussion Our long-standing interest in the chemistry of achiral7,8 and chiral9,10 Ni(II)-complexes of amino acid Schiff bases and their application to the general and scalable11 asymmetric synthesis of α-amino acids led us to the discovery of a modular design12 of nucleophilic glycine and higher amino acid equivalents of general formula 413 (Fig. 2). A major advantage of this modular design is that the careful choice of four basic structural blocks ( phenone, acid, amine and amino acid) provides virtually unlimited structural flexibility, and rational control of the physicochemical properties and reactivity of derivatives 4. Furthermore, Ni(II)-complexes 4 can be assembled and disassembled under operationally convenient conditions and are quite inexpensive. In particular, for the design of reagent 3 Scheme 1 Fig. 2 Modular design of a new generation of Ni(II)-complexes of amino acids. (Fig. 1) we use α-( phenyl)ethylamine, the most inexpensive and readily available, in both enantiomeric forms, chiral auxiliary.14 According to a previous study,6 upon complexation with α-amino acids, (R)-configured reagent 3 gives preference to α-(S) absolute configuration of the corresponding amino acid residues. Therefore, for the interconversion of natural (S)amino acid to (R)-enantiomers, we used reagent 3 with the opposite (S) configuration (Scheme 1). Furthermore, one of the issues to be improved was the slow reaction rates of reagent 3 with amino acids, requiring several days for complete consumption of 3. We found that the application of absolute EtOH, K2CO3 and Ni(OAc)2·4H2O instead of KOH and Ni(NO3)2·6H2O, respectively, allowed for a noticeable increase of the reaction rates. Thus, the reactions of reagent (S)-3 with unbranched amino acids (S)-5a–d were completed within 24 h (Table 1, entries 1–4) affording the products 6a–d with >84% yields. By analogy with the previous results,6 the thermodynamically controlled absolute configuration of the stereogenic centers in compounds 6a–d were assigned to be (S)(SN)(R). Further confirmation of the α-(R) configuration of the amino acids in complexes 6a–d came from the chiroptical properties of free amino acids 7a–d which matched the literature data reported for α-(R) amino acids 7a–d. The reaction of (S)-phenylalanine 5e with reagent (S)-3 gave very similar results (entry 5) to Reactions of reagent (S)-3 with (S)-amino acids to yield (R)-amino acids. 4504 | Org. Biomol. Chem., 2013, 11, 4503–4507 This journal is © The Royal Society of Chemistry 2013 View Article Online Organic & Biomolecular Chemistry Table 1 Paper Reactions of reagent (S)-3 with amino acids (S)-5a–j 6a–j 7a–j a Entry R Base Yield (%) de (%) Yield (%) eeb (%) 1 2 3 4 5 6 7 8 9 10 Me (a) Et (b) n-Pr (c) n-Bu (d) Bn (e) i-Pr (f) i-Bu (g) (CH2)2SMe (h) (CH2)2OH (i) (CH2)2CONH2 ( j) K2CO3 K2CO3 K2CO3 K2CO3 K2CO3 K2CO3 K2CO3 K2CO3 DBU DBU 87 93 88 84 83 47 53 95 93 59 >95 >95 >95 >95 >95 ∼40 ∼70 >95 >95 >95 71 68 65 72 71 N/A N/A 88 84 N/A 97 97 98 98 99 N/A N/A 98 99 N/A Published on 13 May 2013. Downloaded on 18/02/2014 13:49:00. a standard reaction conditions.17 In particular, in the case of unbranched amino acids this approach has an obvious synthetic advantage over the literature methods in terms of the practicality, cost structure and stereochemical outcome. On the other hand, the presented method has some limitations and cannot be applied to highly sterically constrained amino acids as well as to derivatives bearing functional groups incompatible with the basic reaction conditions, capable of coordination to Ni(II). Experimental Determined by 1H NMR analysis of the crude reaction mixtures. b Determined by HPLC analysis, see ref. 16 for the conditions. General procedure for the preparation of Ni(II)-complexes 6a–j by the reaction of (S)-N-(2-benzoylphenyl)-2,2-dimethyl-2-(1phenyl-ethylamino)acetamide (S)-3 with the corresponding amino acids (S)-5a–j that obtained for the unbranched amino acids 5a–d, allowing preparation of the corresponding (R)-amino acid 7e in good overall yield. By contrast, the reactions of bulkier amino acids valine 5f (entry 6) and leucine 5e (entry 7) occurred at relatively slow rates and provided incomplete diastereoselectivity (40% and 70% de, respectively). Of particular interest were the reactions of functionalized (S)-amino acids methionine 5h, homoserine 5i and glutamine 5j. In the case of (S)-methionine 5h, the standard reaction conditions can be used, as in the case of non-functionalized derivatives 5a– g, allowing preparation of the thermodynamically controlled diastereomer 6h and (R)-7h, after disassembly of the complex, in good chemical yield and enantiomeric purity (entry 8). On the other hand, the application of K2CO3 as a base in the reactions of reagent (S)-3 with homoserine (S)-5i and glutamine (S)-5j resulted in the formation of substantial amounts of byproducts and low yields of the target products 6i,j. However, the use of DBU as a base for these reactions remedied this problem and allowed for the preparation of homoserine-containing complex 6i in high chemical yield (entry 9). The DBU-catalyzed reaction of glutamine (S)-5j (entry 10) with reagent (S)-3 was less successful (59% yield), probably due to the partial hydrolysis of the amide functionality. Extension of this method to other functionalized amino acids as well as sterically constrained α-quaternary derivatives was much less successful, thus demonstrating the limitations in the reactivity and applications of reagent 3. Enantiomerically pure samples of (R)-amino acids 7 can be obtained by recrystallization or SDE (self-disproportionation of enantiomers) via achiral chromatography.15 To a flask containing an ethanol solution of reagent (S)-3 (1 eq.), Ni(OAc)2·4H2O (4 eq.) and racemic amino acid (2.0 eq.) was added K2CO3 (15 eq.), and the reaction mixture was stirred at 60–70 °C. Note: in the case of the reactions of homoserine (S)-5i and glutamine (S)-5j, DBU was used as the base instead of K2CO3. The progress of the reaction was monitored by TLC, and upon completion (consumption of reagent 3), the reaction mixture was poured into ice water. The target product was extracted three times with CH2Cl2. The combined organic layer was dried over anhydrous MgSO4 and evaporated in vacuum. After the evaporation of the solvents and silica-gel column chromatography, the target complexes 6a–j were obtained in diastereomerically pure forms. Ni(II) complex of (R)-alanine Schiff base with (S)-N-(2-benzoylphenyl)-2,2-dimethyl-2-(1-phenyl-ethylamino)-acetamide 1 6a. M.p. 270.2 °C (decomp.). [α]25 D = −756.0 (c 1.01, CHCl3). H NMR (300 MHz, CDCl3): δ 1.42 (3 H, d, J = 7.0 Hz), 1.58 (3 H, s), 1.68 (3 H, d, J = 6.7 Hz), 2.53 (1 H, bs), 2.95 (3 H, s), 3.80 (1 H, q, J = 7.0 Hz), 3.89 (1 H, m), 6.58 (1 H, m), 6.66 (1 H, m), 6.84 (1 H, bd, J = 7.9 Hz), 7.04–7.15 (2 H, m), 7.17–7.28 (4 H, m), 7.39–7.57 (3 H, m), 8.04 (1 H, d, J = 8.8 Hz), 8.21 (2 H, bd, J = 7.3 Hz). 13C NMR (75.5 MHz, CDCl3): δ 21.0, 22.1, 22.6, 33.3, 57.5, 64.9, 65.1, 120.2, 123.4, 126.8, 127.4, 127.6, 128.2, 128.6, 128.7, 129.2, 129.4, 131.7, 132.8, 133.5, 140.3, 142.2, 169.6, 180.4, 180.9. HRMS: m/z calcd for C28H29N3NaNiO3 [M + Na]+ 536.1460, found 536.1418. Ni(II) complex of (R)-2-aminobutyric acid Schiff base with (S)-N-(2-benzoylphenyl)-2,2-dimethyl-2-(1-phenyl-ethylamino)acetamide 6b. M.p. 277.5 °C (decomp.). [α]25 D = −983.5 (c 1.07, CHCl3). 1H NMR (300 MHz, CDCl3): δ 1.37 (3 H, m), 1.56 (3 H, s), 1.61–1.75 (2 H, m), 1.66 (3 H, d, J = 6.9 Hz), 2.70–3.00 (1 H, m), 2.87 (3 H, s), 3.82 (1 H, dd, J = 6.8, 4.1 Hz), 3.87 (1 H, dd, J = 6.9, 3.1 Hz), 6.54–6.66 (2 H, m), 6.85 (1 H, m), 7.01–7.12 (2 H, m), 7.13–7.25 (3 H, m), 7.35–7.56 (3 H, m), 8.06 (1 H, dd, J = 8.7, 1.7 Hz), 8.24 (2 H, bd, J = 7.5 Hz). 13C NMR (75.5 MHz, CDCl3): δ 9.58, 22.3, 23.3, 27.5, 33.3, 57.8, 65.3, 69.9, 120.4, 123.4, 126.8, 127.1, 127.6, 127.9, 128.5, 128.8, 128.9, 129.3, 129.6, 132.0, 133.2, 134.1, 140.4, 142.5, 170.1, 179.8, 180.4. Conclusions In conclusion, this work has demonstrated that various (S)α-amino acids can be interconverted to the corresponding (R)α-enantiomers via reaction with chiral reagent (S)-3 followed by disassembly of the intermediate Ni(II)-complexes under This journal is © The Royal Society of Chemistry 2013 Org. Biomol. Chem., 2013, 11, 4503–4507 | 4505 View Article Online Published on 13 May 2013. Downloaded on 18/02/2014 13:49:00. Paper HRMS: m/z calcd for C29H32N3NiO3 [M + H]+ 528.1797, found 528.1666. Ni(II) complex of (R)-2-aminopentanoic acid Schiff base with (S)-N-(2-benzoylphenyl)-2,2-dimethyl-2-(1-phenyl-ethylamino)acetamide 6c. M.p. 293.5 °C (decomp.). [α]25 D = −973.5 (c 1.05, CHCl3). 1H NMR (300 MHz, CDCl3): δ 1.33 (3 H, m), 1.56 (3 H, s), 1.58–1.77 (4 H, m), 1.65 (3 H, d, J = 6.9 Hz), 2.69–2.92 (1 H, m), 2.88 (3 H, s), 3.81 (1 H, dd, J = 6.8, 4.1 Hz), 3.88 (1 H, dd, J = 6.9, 3.1 Hz), 6.55–6.66 (2 H, m), 6.84 (1 H, m), 7.00–7.12 (2 H, m), 7.12–7.26 (3 H, m), 7.35–7.57 (3 H, m), 8.05 (1 H, dd, J = 8.7, 1.7 Hz), 8.23 (2 H, bd, J = 7.5 Hz). 13C NMR (75.5 MHz, CDCl3): δ 9.55, 18.9, 22.5, 23.2, 28.6, 33.9, 59.8, 65.9, 71.2, 121.5, 123.9, 127.3, 126.9, 127.8, 128.4, 128.6, 128.8, 128.9, 129.2, 129.8, 132.7, 133.4, 134.0, 141.0, 142.1, 171.2, 178.7, 180.3. HRMS: m/z calcd for C30H34N3NiO3 [M + H]+ 542.1954, found 542.1911. Ni(II) complex of (R)-2-aminohexanoic acid Schiff base with (S)-N-(2-benzoylphenyl)-2,2-dimethyl-2-(1-phenyl-ethylamino)acetamide 6d. M.p. 250.0 °C (decomp.). [α]25 D = −893.7 (c 1.03, CHCl3). 1H NMR (300 MHz, CDCl3): δ 1.32 (3 H, m), 1.56 (3 H, s), 1.57–1.80 (6 H, m), 1.65 (3 H, d, J = 6.9 Hz), 2.71–2.99 (1 H, m), 2.88 (3 H, s), 3.81 (1 H, dd, J = 6.8, 4.1 Hz), 3.88 (1 H, dd, J = 6.9, 3.1 Hz), 6.55–6.67 (2 H, m), 6.84 (1 H, m), 7.01–7.13 (2 H, m), 7.12–7.26 (3 H, m), 7.35–7.55 (3 H, m), 8.05 (1 H, dd, J = 8.7, 1.7 Hz), 8.25 (2 H, bd, J = 7.5 Hz). 13C NMR (75.5 MHz, CDCl3): δ 9.51, 20.5, 22.3, 23.2, 27.4, 33.5, 56.8, 66.5, 70.3, 121.2, 123.3, 126.5, 127.2, 127.8, 127.9, 128.5, 128.8, 128.9, 129.3, 129.6, 132.1, 133.2, 134.2, 140.4, 142.5, 170.7, 179.3, 180.5. HRMS: m/z calcd for C31H36N3NiO3 [M + H]+ 556.2110, found 556.2012. Ni(II) complex of (R)-phenylalanine Schiff base with (S)-N-(2benzoylphenyl)-2,2-dimethyl-2-(1-phenyl-ethylamino)-acetamide 6e. M.p. 281 °C (decomp.). [α]25 D = −501.3 (c 1.08, CHCl3). 1H NMR (300 MHz, CDCl3): δ 1.58 (3 H, d, J = 7.0 Hz), 2.17 (6 H, s), 2.22 (1 H, bs), 2.68 (1 H, dd, J = 13.8, 5.6 Hz), 3.07 (1 H, dd, J = 13.8, 4.4 Hz), 3.75 (1 H, qd, J = 7.0, 3.2 Hz), 4.11 (1 H, dd, J = 5.6, 4.4 Hz), 6.59 (1 H, dd, J = 8.2, 2.0 Hz), 6.64 (1 H, ddd, J = 8.2, 6.4, 1.17 Hz), 7.02–7.61 (14 H, m), 8.09 (2 H, bd, J = 7.3 Hz), 8.17 (1 H, dd, J = 8.8, 1.2 Hz). 13C NMR (75.5 MHz, CDCl3): δ 22.1, 23.1, 32.8, 39.1, 57.4, 64.9, 70.1, 120.1, 123.0, 126.7, 126.9, 127.2, 127.7, 127.8, 128.3, 128.7, 128.9, 129.1, 129.6, 130.1, 132.1, 133.3, 134.1, 135.5, 140.3, 142.9, 170.5, 179.0, 180.2. HRMS: m/z calcd for C34H33N3NiO3 [M + H]+ 612.1773, found 612.1725. Ni(II) complex of (R)-valine Schiff base with (R)-N-(2-benzoylphenyl)-2,2-dimethyl-2-(1-phenyl-ethylamino)-acetamide 6f. M.p. 294.5 °C (decomp.). [α]25 D = −2043.0 (c 1.10, CHCl3). 1 H NMR (300 MHz, CDCl3): δ 0.73 (3 H, d, J = 6.7 Hz), 1.56 (3 H, s), 1.65–1.85 (1 H, m), 1.69 (3 H, d, J = 6.9 Hz), 1.75 (3 H, d, J = 6.2 Hz), 2.83 (3 H, s), 3.08 (1 H, bd, J = 2.3 Hz), 3.66 (1 H, d, J = 3.1 Hz), 3.86 (1 H, m), 6.55–6.66 (2 H, m), 6.85 (1 H, bd, J = 7.7 Hz), 7.02 (1 H, m), 7.05 (1 H, m), 7.12–7.18 (2 H, m), 7.22 (1 H, bd, J = 7.2 Hz), 7.42 (1 H, m), 7.43–7.57 (2 H, m), 8.15 (1 H, d, J = 8.5 Hz), 8.22 (2 H, bd, J = 7.4 Hz). 13C NMR (75.5 MHz, CDCl3): δ 17.7, 19.8, 22.1, 23.1, 32.8, 34.1, 57.7, 65.0, 73.8, 120.1, 123.0, 126.9, 129.9, 127.6, 127.9, 128.2, 128.5, 4506 | Org. Biomol. Chem., 2013, 11, 4503–4507 Organic & Biomolecular Chemistry 128.8, 129.3, 129.3, 131.9, 133.2, 134.1, 140.6, 142.6, 170.0, 178.1, 180.8. HRMS: m/z calcd for C30H34N3NiO3 [M + H]+ 542.1954, found 542.1964. Ni(II) complex of (R)-amino-4-methylpentanoic acid Schiff base with (S)-N-(2-benzoylphenyl)-2,2-dimethyl-2-(1-phenylethylamino)-acetamide 6g. M.p. 274.5 °C (decomp.). [α]25 D = −1011.9 (c 1.12, CHCl3). 1H NMR (300 MHz, CDCl3): δ 0.76 (3 H, d, J = 6.7 Hz), 0.77 (3 H, d, J = 6.7 Hz), 1.01 (1 H, m), 1.56 (3 H, s), 1.55–1.70 (2 H, m), 1.65 (3 H, d, J = 6.9 Hz), 2.88 (3 H, s), 3.84 (1 H, m), 3.88 (1 H, m), 6.53–6.65 (2 H, m), 6.85 (1 H, m), 6.98–7.11 (2 H, m), 7.14–7.25 (3 H, m), 7.36–7.55 (3 H, m), 8.04 (1 H, dd, J = 8.7, 1.7 Hz), 8.22 (2 H, bd, J = 7.5 Hz). 13C NMR (75.5 MHz, CDCl3): δ 9.55, 9.56, 18.7, 22.3, 23.7, 29.1, 34.7, 50.2, 66.2, 71.8, 122.0, 123.8, 127.4, 126.8, 127.9, 128.5, 128.6, 128.8, 128.9, 129.3, 130.1, 132.5, 133.3, 134.0, 141.4, 141.9, 172.3, 179.9, 181.0. HRMS: m/z calcd for C31H36N3NiO3 [M + H]+ 556.2110, found 556.2743. Ni(II) complex of (R)-2-amino-4-(methylthio)butanoic acid Schiff base with (S)-N-(2-benzoylphenyl)-2,2-dimethyl-2-(1phenyl-ethylamino)-acetamide 6h. M.p. 230.1 °C (decomp.). 1 [α]25 D = −951.0 (c 1.04, CHCl3). H NMR (300 MHz, CDCl3): δ 1.58 (3 H, s), 1.68 (3 H, d, J = 6.7 Hz), 1.81–2.18 (2 H, m), 2.19 (3 H, s), 2.45–2.52 (1 H, m), 2.53 (1 H, bs), 2.95 (3 H, s), 3.18–3.35 (1 H, m), 3.88 (1 H, m), 6.57 (1 H, m), 6.66 (1 H, m), 6.85 (1 H, bd, J = 7.9 Hz), 7.05–7.15 (2 H, m), 7.17–7.27 (4 H, m), 7.38–7.57 (3 H, m), 8.05 (1 H, d, J = 8.8 Hz), 8.22 (2 H, bd, J = 7.3 Hz). 13C NMR (75.5 MHz, CDCl3): δ 17.8, 22.1, 22.6, 30.1, 33.3, 33.9, 57.6, 65.0, 65.5, 120.7, 123.5, 126.7, 127.3, 127.6, 128.4, 128.6, 128.8, 129.2, 129.5, 131.8, 132.6, 133.7, 140.9, 143.4, 170.1, 180.7, 181.2. HRMS: m/z calcd for C30H34N3NiO3S [M + H]+ 574.1674, found 574.1593. Ni(II) complex of (R)-2-amino-4-hydroxybutanoic acid Schiff base with (S)-N-(2-benzoylphenyl)-2,2-dimethyl-2-(1-phenylethylamino)-acetamide 6i. M.p. 293.7 °C (decomp.). [α]25 D = −830.0 (c 0.98, CHCl3). 1H NMR (300 MHz, CDCl3): δ 1.58 (3 H, s), 1.68 (3 H, d, J = 6.7 Hz), 1.99 (2 H, m), 2.11 (1 H, m), 2.53 (1 H, bs), 2.95 (3 H, s), 3.41–3.67 (3 H, m), 3.92 (1 H, m), 6.56 (1 H, m), 6.68 (1 H, m), 6.84 (1 H, bd, J = 7.9 Hz), 7.03–7.16 (2 H, m), 7.16–7.28 (4 H, m), 7.40–7.55 (3 H, m), 8.05 (1 H, d, J = 8.8 Hz), 8.20 (2 H, bd, J = 7.3 Hz). 13C NMR (75.5 MHz, CDCl3): δ 22.1, 22.6, 33.3, 38.3, 57.6, 58.2, 64.7, 64.9, 120.3, 123.5, 127.0, 127.4, 127.68, 128.1, 128.5, 128.7, 129.3, 129.8, 131.5, 133.0, 133.7, 140.1, 142.9, 169.1, 180.9, 181.0. HRMS: m/z calcd for C29H32N3NiO4 [M + H]+ 544.1746, found 544.1672. Ni(II) complex of (R)-2-amino-4-carbamoylbutanoic acid (glutamine) Schiff base with (S)-N-(2-benzoylphenyl)-2,2-dimethyl2-(1-phenyl-ethylamino)-acetamide 6j. M.p. 297.5 °C. [α]25 D = −936.0 (c 1.08, CHCl3). (decomp.). 1H NMR (300 MHz, CDCl3): δ 1.55 (3 H, s), 1.68 (3 H, d, J = 6.7 Hz), 2.15 (2 H, m), 2.24 (2 H, m), 2.60 (1 H, bs), 2.94 (3 H, s), 3.78 (1 H, m), 3.91 (1 H, m), 6.28 (2 H, bs), 6.55 (1 H, m), 6.69 (1 H, m), 6.81 (1 H, bd, J = 7.9 Hz), 7.07–7.16 (2 H, m), 7.15–7.27 (4 H, m), 7.40–7.55 (3 H, m), 8.05 (1 H, d, J = 8.8 Hz), 8.20 (2 H, bd, J = 7.3 Hz). 13C NMR (75.5 MHz, CDCl3): δ 22.4, 23.2, 29.5, 33.0, 33.3, 57.6, 64.7, 65.4, 120.1, 123.5, 126.8, 127.4, 127.6, 128.3, 128.6, 128.8, 129.3, 129.8, 131.6, 132.5, 134.0, 140.4, 142.5, 170.0, 175.9, This journal is © The Royal Society of Chemistry 2013 View Article Online Organic & Biomolecular Chemistry 180.7, 181.3. HRMS: m/z calcd for C30H33N4NiO4 [M + H]+ 571.1855, found 571.1798. Published on 13 May 2013. Downloaded on 18/02/2014 13:49:00. General procedure for the decomposition of complexes 6a–j, isolation of target amino acids 7a–j and recovery of chiral reagent (S)-3 A solution of diastereomerically pure complexes 6a–j (25 mmol) in MeOH (50 mL) was added to a stirring solution of 3 N HCl in MeOH at 70 °C. Upon the disappearance of the red color (about 5–10 min), the reaction mixture was evaporated under vacuum. Water (85 mL) was added and the resulting mixture was treated with an excess of concentrated NH4OH and extracted with CH2Cl2 or CHCl3. The organic extracts were dried over magnesium sulfate and evaporated in vacuum to give (>95%) ligand (S)-3. The aqueous solution was evaporated in vacuum, dissolved in a minimum amount of water, and passed through the cation-exchange resin Dowex 50X2 100 to afford analytically pure samples of the target amino acids (91–95%) 7a–j. Acknowledgements We thank IKERBASQUE, Basque Foundation for Science, the Basque Government (SAIOTEK S-PE12UN044) and Hamari Chemicals (Osaka, Japan) for generous financial support. Notes and references 1 (a) L. Stryer, Biochemistry, W. H. Freeman and Co., New York, 4th edn, 2000; (b) G. Kreil, Annu. Rev. Biochem., 1997, 66, 337–345. 2 D-Amino Acids: A New Frontier in Amino Acids and Protein Research – Practical Methods and Protocols, ed. R. Konno, H. Brückner, A. D’Aniello, G. Fischer, N. Fujii and H. Homma, Nova Science Publishers, Inc., New York, 2007. 3 (a) Y. Ikenaka and S. Takahashi, Encycl. Ind. Biotechnol., 2010, 1, 226–235; (b) M. Wakayama, K. Yoshimune, Y. Hirose and M. Moriguchi, J. Mol. Catal. B: Enzym., 2003, 23, 71–85; (c) R. Sharma and R. M. Vohra, Curr. Sci., 1999, 77, 127–136; (d) M. Yagasaki and A. Ozaki, J. Mol. Catal. B: Enzym., 1998, 4, 1–11. 4 A. Solladié-Cavallo, O. Sedy, M. Salisova and M. Schmitt, Eur. J. Org. Chem., 2002, 3042–3049. 5 H. Park, K. M. Kim, A. Lee, S. Ham, W. Nam and J. Chin, J. Am. Chem. Soc., 2007, 129, 1518–1519. 6 (a) V. A. Soloshonok, T. K. Ellis and H. Ueki Ono, J. Am. Chem. Soc., 2009, 131, 7208–7209; (b) A. E. Sorochinsky, H. Ueki, J. L. Aceña, T. K. Ellis, H. Moriwaki, T. Sato and V. A. Soloshonok, J. Fluorine Chem., 2013, DOI: 10.1016/ j.jfluchem.2013.02.022, in press. This journal is © The Royal Society of Chemistry 2013 Paper 7 For large-scale synthesis of achiral glycine equivalents, see: H. Ueki, T. K. Ellis, C. H. Martin and V. A. Soloshonok, Eur. J. Org. Chem., 2003, 1954–1957. 8 (a) V. A. Soloshonok, C. Cai and V. J. Hruby, Tetrahedron: Asymmetry, 1999, 10, 4265–4269; (b) V. A. Soloshonok, C. Cai and V. J. Hruby, Tetrahedron Lett., 2000, 41, 9645– 9649; (c) T. K. Ellis, C. H. Martin, G. M. Tsai, H. Ueki and V. A. Soloshonok, J. Org. Chem., 2003, 68, 6208–6214. 9 For large-scale synthesis of chiral glycine equivalents, see: H. Ueki, T. K. Ellis, C. H. Martin, S. B. Bolene, T. U. Boettiger and V. A. Soloshonok, J. Org. Chem., 2003, 68, 7104–7107. 10 (a) X. Tang, V. A. Soloshonok and V. J. Hruby, Tetrahedron: Asymmetry, 2000, 11, 2917–2925; (b) W. Qiu, V. A. Soloshonok, C. Cai, X. Tang and V. J. Hruby, Tetrahedron, 2000, 56, 2577–2582; (c) V. A. Soloshonok, X. Tang, V. J. Hruby and L. Van Meervelt, Org. Lett., 2001, 3, 341– 343. 11 (a) V. A. Soloshonok, X. Tang and V. J. Hruby, Tetrahedron, 2001, 57, 6375–6382; (b) V. A. Soloshonok, H. Ueki, R. Tiwari, C. Cai and V. J. Hruby, J. Org. Chem., 2004, 69, 4984–4990; (c) V. A. Soloshonok, C. Cai and V. J. Hruby, Org. Lett., 2000, 2, 747–750; (d) S. M. Taylor, T. Yamada, H. Ueki and V. A. Soloshonok, Tetrahedron Lett., 2004, 45, 9159–9162; (e) J. Wang, D. Lin, S. Zhou, X. Ding, V. A. Soloshonok and H. Liu, J. Org. Chem., 2011, 76, 684– 687. 12 (a) V. A. Soloshonok, H. Ueki and T. K. Ellis, Tetrahedron Lett., 2005, 46, 941–944; (b) V. A. Soloshonok, H. Ueki, T. K. Ellis, T. Yamada and Y. Ohfune, Tetrahedron Lett., 2005, 46, 1107–1110; (c) T. K. Ellis, H. Ueki, T. Yamada, Y. Ohfune and V. A. Soloshonok, J. Org. Chem., 2006, 71, 8572–8578. 13 (a) V. A. Soloshonok, H. Ueki and T. K. Ellis, Synlett, 2009, 704–715; (b) V. A. Soloshonok and T. K. Ellis, Synlett, 2006, 533–538; (c) T. Yamada, T. Okada, K. Sakaguchi, Y. Ohfune, H. Ueki and V. A. Soloshonok, Org. Lett., 2006, 8, 5625– 5628; (d) V. A. Soloshonok, H. Ueki and T. K. Ellis, Chim. Oggi/Chem. Today, 2008, 26, 51–54. 14 (a) E. Juaristi, J. Escalante, J. L. León-Romo and A. Reyes, Tetrahedron: Asymmetry, 1998, 9, 715–740; (b) E. Juaristi, J. L. León-Romo, A. Reyes and J. Escalante, Tetrahedron: Asymmetry, 1999, 10, 2441–2495. 15 (a) T. Nakamura, K. Tateishi, S. Tsukagoshi, S. Hashimoto, S. Watanabe, V. A. Soloshonok, J. L. Aceña and O. Kitagawa, Tetrahedron, 2012, 68, 4013–4017; (b) V. A. Soloshonok and D. O. Berbasov, Chim. Oggi/Chem. Today, 2006, 24, 44–47. 16 V. A. Soloshonok, A. G. Kirilenko, S. V. Galushko and V. P. Kukhar, Tetrahedron Lett., 1994, 35, 5063–5064. 17 V. A. Soloshonok, C. Cai, T. Yamada, H. Ueki, Y. Ohfune and V. J. Hruby, J. Am. Chem. Soc., 2005, 127, 15296–15303. Org. Biomol. Chem., 2013, 11, 4503–4507 | 4507