1 Justification and Protocol for Extended-Infusion
advertisement

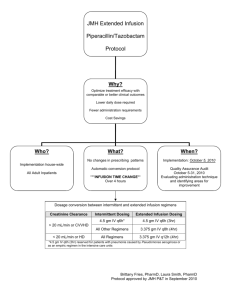
JustificationandProtocolforExtended‐InfusionPiperacillin/Tazobactam inAdultPatients version2/5/13[DRAFT] EXECUTIVESUMMARY 1. Useofextendedinfusions(EI)ofpiperacillin‐tazobactam(PTZ)optimizestheprobabilitythatdrug concentrations will remain above the minimum inhibitory concentration (MIC) for an appropriate periodoftime. 2. ThegoalsoftheextendedinfusionPTZprotocolaretooptimizetreatmentoutcomes,whilecurtailing resistancetoPTZandreducingcost. 3. We recommend implementing the extended infusion protocol in all adult patients to realize both clinical and economic advantages. It was noted that a number of Pseudomonas aeruginosa isolatesat<<HOSPITAL>>haveMICs>8mcg/mLandtheextendedinfusionprotocolwouldofferan advantageinthetreatmentofthesecases. BACKGROUND Piperacillin/tazobactam is a β‐lactam/ β‐lactamase inhibitor combination formulary antibiotic frequently usedatXXXHospitalforthetreatmentofmoderate‐to‐severeinfections,includingsepsis,lowerrespiratory tract, urinary tract, complicated skin and skin structure, gynecologic, bone and joint, and intra‐abdominal infections.Overall,PTZisoneofthemostcommonlyusedantibioticsat<<HOSPITAL>>. Increasing antimicrobial resistance and the resulting increased mortality has led to the reevaluation of the optimal method to administer antibiotics. Studies have shown that for β‐lactams (including PTZ), the best predictor of bacterial killing is the time during which the free drug concentration exceeds the minimum inhibitory concentration of the organism (%fT>MIC). Specifically, maximal bactericidal effect is achieved when free drug concentration exceeds the MIC by approximately four‐fold for 40% to 60% of the dosing interval.1,2 JUSTIFICATION Pharmacodynamic studies. Several studies have explored the PK/PD parameters of PTZ with the goal of optimizing its clinical utility. These studies have focused on strategies to enhance the duration of drug exposure.Inonesuchstudy(illustratedinFigure1),theprobabilitiesoftargetattainmentof50%fT>MIC forPTZwereasfollows: 3.375gq6hr(0.5‐hrinfusion):>90%forMICvalues≤1mg/L 3.375gq4hr(0.5‐hrinfusion):>90%forMICvaluesupto8mg/L 3.375gq8h(4‐hrinfusion):>90%forMICsupto16mg/L 1 Figure1:P Probabilitieso oftargetattain nmentwithPT TZ4 MIC = minimum m inhibitory conce entration ptofourtimessdaily)failedtoprovidead dequatetime above Asillustraatedabove,traaditionaldosingofPTZ(up theMICfo ororganismsw withanMIC>8mg/L.3,4No oneofthereg imenswerereeliableatanM MICof64mg//L(the susceptibiilitybreakpoin nt).Alternativ vely,adoseoff3.375gq8hr infusedover 4hoursproviidesaprobabiilityof target atttainment >90% for an MIIC up to 16 mg/L at a loower total d aily dose thaan standard doses. Furthermo ore,PTZlackssanypersisten nteffects(posst‐antibioticeeffect)thatlasstafterantimicrobialexposureto mostorgaanisms,suchth hatoncetheffreedrugconccentrationsfalllbelowtheM MIC,bacterialrre‐growthisaalmost instantaneeous. Extend ding the admiinistration tim me takes advaantage of the pharmacodyn namic propertties of thisextendedspectrum mβ‐lactam/β‐lactamaseinhiibitorcombinaation. t MICs of PTZ P against P. P aeruginosa isolates was conducted w with <<HOSPIT TAL>> An internal review of the ogy data from m January 201 11 to Decemb ber 2011. Acccording to th he Clinical Lab boratory Stan ndards microbiolo Institute (CLSI) ( guideliines, the breaakpoint for su usceptibility oof P. aeruginoosa is ≤64 mgg/L. Results of the internalsu urveyareillusstratedinTable1and2belo ow: aPTZMIC;201 11 Table1:CombinedICU:P.aeruginosa Susceptible In ntermediate/ Resistant MIC ≤8 16 32 2 6 64 >64 Totall #isolates 82 11 3 7 21 124 Allhospitalisolates:P.aerug ginosaPTZMIC;2011 Table2:A In ntermediate/ Susceptible Resistant MIC ≤8 16 6 32 3 64 >64 Totall #isolates 348 48 8 10 1 38 65 509 h may potentiaally be sub‐op ptimally treatted by These datta reveal thatt 22% (n=96) of isolates hospital‐wide traditionaaladministratiionofPTZ.Continuingwith hthecurrentp practicecould dresultinaneegativeoutcom mefor a number of patients per p year cared d for at <<HO OSPITAL>>. Booth ICU and n non‐ICU settin ngs are affectted by drugsusceptib bility,withsev veralpatientsaffectedonth henon‐ICUgeeneralwards. impairedd c relevaance of these findings has also been in nvestigated. In one such study, Clinical sttudies. The clinical outcomes were examined in patien nts before an nd after imp lementation of a hospitall‐wide substitution 2 programwhereintermittentlyinfusedPTZwasautomaticallyconvertedtoEI.5Inpatientsatgreatestrisk formortality(APACHEIIscore≥17)EIPTZresultedinsignificantlylower14‐daymortalityrates(12.2%vs. 31.6%, p=0.04) and median hospital length of stay (LOS) (21 days vs. 38 days, p=0.02) compared with patientswhoreceivedintermittentinfusionPTZ. Furthermore,in a recentmeta‐analysis,the authors noteda reduced mortality amongpatientsreceiving β‐ lactamagentsadministeredbyEIcomparedtothosereceivingintermittentinfusions.6 Economic evaluation. In the previous study, EI PTZ resulted in reduction of total daily dose by 25%‐50% representingconsiderablesavingsinannualdirectdrugacquisitioncosts.5 Projected expenditures for automatic therapeutic interchange to PTZ 3.375g (infused over 4 hrs) q8h at <<HOSPITAL>>aresummarizedinTable3.BasedonPTZutilizationdatafromfiscalyear2012,aprojected costsavingsofapproximately$150,000mayberealizedwithaswitchtoextendedinfusionPTZ. Table3.ProjectedFinancialImpactofPTZProlongedInfusionat<<HOSPITAL>>inFY2013 PTZDose Cost/day/patient FY13Total 3.375gIVq6h $44 $602,250 3.375gIVq8h $33 $451,688 Difference $11 $150,562 ALTERNATEDOSINGPROPOSAL: Patientpopulationsimpacted:Adultpatientshospitalizedat<<HOSPITAL>>onadultwards Procedure:Ordersforstandarddoses/administrationofPTZforadultswillbeinterchangedwithextended‐ infusion as described in Table 4. Pharmacists will assist providers in adjusting the dose of PTZ for renal functionasindicatedinthechartbelow: Table4.AdjustmentofEIPiperacillin/tazobactamDosingBasedonRenalFunction4,7 Clcr(mL/min) Over20mL/min ≤20mL/min(including ContinuousRenal peritonealorhemodialysis) ReplacementTherapy (CRRT) Pip/TazoDose 3.375gIVover4hours 3.375gIVover4hours 3.375gIVover4hoursq8h q8h q12h ExceptionstothePolicy Doseadjustments:Certainpopulationswithenhanceddrugclearance,suchascriticallyillpatientswithsevere infectionsorpatientswithcysticfibrosis,maybenefitfrommoreintensivePTZdosingtomaximize%fT>MIC. However,doseintensificationstudiesinthesepopulationsarelimitedandrequirefurtherelucidation.Itis notrecommendedtoincreasethedosebeyond3.375gq8hwithoutcarefulconsiderationofpatient‐specific factorsincludingseverityofillnessandMICoftheorganism. EmergencyDepartment(pre‐admissionstatusonly),ORandPACUareas,andambulatoryclinics:Patientswith orders for PTZ from these areas for which 4‐hour infusion times may not be practical may receive a traditionalstandard30‐minuteinfusionatthediscretionofthephysicianand/ornursingstaff.Subsequent extended‐infusiondosesshouldbeadministered6‐8hoursaftertheintermittentdose. Medicationschedulingand/ordrugcompatibilityconflicts:Clinicalpharmacistswillalsobeavailabletoassist nursing to help resolve medication scheduling and compatibility issues (see Appendix A). If shifting medication administration times does not alleviate the problem, nursing may request standard 30‐min 3 infusionsafterconsultingwiththepharmacistandphysician.Placingnewlinestoaccommodateextended‐ infusion PTZ is discouraged, and should only be done upon the discretion of the attending physician or InfectiousDiseasesConsultTeaminwhichthebenefitsarebelievedtooutweighanyrisks. IMPLEMENTATIONTIMELINE: Extended‐InfusionPTZImplementationComponent SuggestedTime EIPTZProtocolASETreview February2013 EIPTZProtocolP&Treview March2013 Pharmacisteducation March2013–April2013 Nursing/physicianeducation(allow4‐6weekspriortoimplementation forpharmacist‐leadinservicepresentationsanddistributionof educationalhandouts) E‐browserCPOE/HMMimplementation April15,2013 EPICimplementation June22,2013 6monthauditandfeedbackdatacollection Sept2013 REFERENCES: 1. Craig WA, Ebert SC. Continuous infusion of beta‐lactam antibiotics. Antimicrob Agents Chemother 1992; 36:2577‐2583. 2.GrantE,NicolauDP,NightingaleCH,QuintilianiR.Continuousinfusionofbeta‐lactamantibiotics.ConnMed 1999;63:275‐277. 3. Lodise TP, LomaestroB, RodvoldK, DanzigerL, DrusanoG. Pharmacodynamic profiling of piperacillin in the presence of tazobactam in patients through the use of population pharmacokinetic models and Monte Carlosimulation.AntimicrobAgentsChemother2004;48(12):4718‐24. 4. Lodise TP, LomaestroB, DrusanoG. Application of antimicrobial pharmacodynamic concepts into clinical practice:focusonβ‐lactamantibiotics.Pharmacotherapy2006;26(9):1320‐1332. 5.LodiseTP,LomaestroB,DrusanoG.Piperacillin‐tazobactamforPseudomonasaeruginosainfection:clinical implicationsofanextended‐infusiondosingstrategy.ClinInfectDis2007;44(3):357‐63. 6. Falagas ME, Tansarli GS, Ikawa K, Vardakas KZ. Clinical outcomes with continuous or extended versus short‐term intravenous infusion carbapenems and piperacillin/tazobactam: a systematic review and meta‐ analysis.ClinInfectDis2013;56(2):272‐82. 7. Patel N, Scheetz MH, Drusano GL, Lodise TP. Identification of optimal renal dosage adjustments for traditional and extended‐infusion piperacillin/tazobactam dosing regimens in hospitalized patients. AntimicrobAgentsChemother2010;54(1):460‐65. 8. Trissel LA. Piperacillin sodium‐tazobactam sodium. Handbook on Injectable Drugs 16th ed. 2011:1279‐ 1287. January2013 Preparedby:ChristinaSarubbi,Pharm.D. Reviewedby:DeverickAnderson,MD,MPH;RichardDrew,PharmD,MS;RebekahMoehring,MD,MPH; MichelleSharpe,PharmD 4 AppendixA.CompatibilityofSelectDrugswithPTZadministeredbyintermittentorcontinuous infusion8 The standard concentration of PTZ 3.375g mini‐bag with a total volume of 100 mL = 33 mg/mL. More comprehensivelistingsofcompatibleandincompatibledrugsmaybefoundindrug‐dosinghandbooks. Y‐sitecompatible[CanbeY‐sitedtogetherwiththeseintermittentorcontinuousIVmeds] Antibiotics Other GI/Reflux Anticoagulants Amikacin Bivalirudin Pantoprazole Allopurinol Aztreonam Heparin Metoclopramide Digoxin Cefepime Ranitidine Diphenhydramine Clindamycin Magnesiumsulfate Diuretics Metronidazole Mannitol Bumetanide Pressors TMP/SMX(Bactrim®) Methylprednisolone Dopamine Furosemide PotassiumChloride Tigecycline Norepinephrine Potassiumphosphate Phenylepherine Drips Antifungals Vasopressin Fentanyl AmphotericinB Lorazepam liposomal(Ambisome®) Morphine AmphotericinBlipid SodiumBicarbonate complex(Abelcet®) Fluconazole Voriconazole NOT‐COMPATIBLE‐[DoNotPiggybackTogether] Antibiotics Other Other Antivirals Azithromycin Acyclovir Chlorpromazine Haloperidol Ciprofloxacin Diltiazem Insulin,regular Doxycycline Antifungals Dobutamine Promethazine Micafungin Gentamicin Famotidine Nalbuphine Levofloxacin Midazolam Moxifloxacin Propofol Tobramycin Vancomycin** **VariablecompatibilityviaY‐site: Variable compatibility has been reported for vancomycin and PTZ and is concentration‐dependent. Vancomycinconcentrationsatorexceeding20mg/mLareincompatiblewithallconcentrationsofPTZ.PTZ concentrations of 45 mg/mL are compatible with vancomycin concentrations of <=2 mg/mL but this concentration of vancomycin is not typically used. Thus, vancomycin and PTZ should be considered incompatible. 5