Naming Ionic Compounds Slides (pdf file)
advertisement

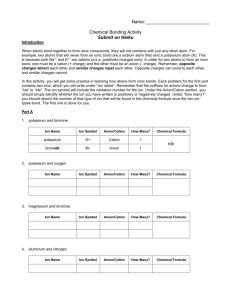
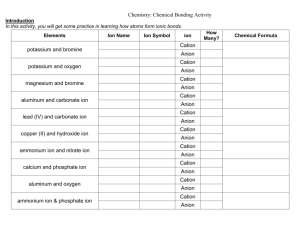
Naming Ionic Compounds Names of Ionic Compounds 1. Identify the ions 2. Name the cation 3. Name the anion The ions are named in the same order that they are written in the formula. Name only one cation and only one anion. Identify the Ions formula Na Cl Al2S3 Fe F3 Cu3(PO4)2 (NH4)2SO3 Each of our ionic compounds will contain one kind of cation and one kind of anion (underlined at left). If the ionic compound has only two elements, each is usually a monatomic ion. If the compound has more than two elements, there is usually at least one polyatomic ion. Identifying Ions Examples formula Notice that subscript numbers are part of the compound formula (white numbers) or are part of the polyatomic ions (colored numbers) cation anion NaCl Na1+ Cl1- Al2S3 Al3+ S2- FeF3 Fe3+ F1- Cu3(PO4)2 Cu2+ PO43- (NH4)2SO3 NH41+ SO32- Ion Names Examples formula cation anion NaCl Na1+ sodium ion Cl1- Al2S3 Al3+ aluminum ion FeF3 Fe3+ iron(III) ion Cu3(PO4)2 Cu2+ copper(II) ion (NH4)2SO3 NH41+ ammonium ion formula cation chloride ion NaCl Na1+ sodium ion Cl1- chloride ion S2- sulfide ion Al2S3 Al3+ aluminum ion S2- sulfide ion F1- fluoride ion FeF3 Fe3+ iron(III) ion F1- fluoride ion PO43- phosphate ion Cu3(PO4)2 Cu2+ copper(II) ion PO43- phosphate ion SO32- sulfite ion (NH4)2SO3 NH41+ ammonium ion SO32- sulfite ion Polyatomic Ions Examples formula cation NaCl Na1+ Monatomic Ion Examples anion sodium ion Cl1- chloride ion Al2S3 Al3+ aluminum ion S2- sulfide ion FeF3 Fe3+ iron(III) ion F1- fluoride ion Cu3(PO4)2 Cu2+ copper(II) ion PO43- phosphate ion (NH4)2SO3 NH41+ ammonium ion SO32- sulfite ion anion Compound Names are formed by combining Ion Names formula cation anion NaCl Al2S3 FeF3 Cu3(PO4)2 (NH4)2SO3 name cation anion sodium chloride aluminum sulfide iron(III) fluoride copper(II) phosphate ammonium sulfite Monatomic Cation Names The name of each monatomic cation is the same as the name of the element. A roman numeral indicates the cation’s charge, when there is more than one possibility. formula cation anion NaCl Al2S3 FeF3 Cu3(PO4)2 (NH4)2SO3 name cation anion sodium chloride aluminum sulfide iron(III) fluoride copper(II) phosphate NaCl Al2S3 There is only one polyatomic cation common in compound naming. That is ammonium ion. FeF3 Cu3(PO4)2 (NH4)2SO3 The name of each monatomic anion is the same as the name of the element but with an -ide suffix. ammonium sulfite Polyatomic Cation Names formula cation anion Monatomic Anion Names name cation anion sodium chloride aluminum sulfide iron(III) fluoride copper(II) phosphate ammonium sulfite formula cation anion NaCl Al2S3 FeF3 Cu3(PO4)2 (NH4)2SO3 name cation anion sodium chloride aluminum sulfide iron(III) fluoride copper(II) phosphate ammonium sulfite Polyatomic Anion Names There are a numerous polyatomic anions. Most have names with -ate or -ite endings. This always indicates that oxygen is part of the ion. formula cation anion NaCl Al2S3 FeF3 Cu3(PO4)2 (NH4)2SO3 name cation anion sodium chloride aluminum sulfide iron(III) fluoride copper(II) phosphate ammonium sulfite Ion Charges and Naming Ion charges must be used to write ionic formulas, but naming compounds often does not require using their charges. Na1+ sodium ion Cl1- chloride ion Al3+ aluminum ion S2- sulfide ion Fe3+ iron(III) ion F1- fluoride ion Cu2+ copper(II) ion PO43- phosphate ion ammonium ion SO32- sulfite ion NH4 1+ Specifying charge formula To name ionic compounds such as these, we must indicate the charge of the cation. Roman numerals are the simplest way to do this. name FeF2 iron(II) fluoride FeF3 iron(III) fluoride Cu3PO4 copper(I) phosphate Cu3(PO4)2 copper(II) phosphate SnO tin(II) oxide SnO2 tin(IV) oxide When to specify charge Charge affects the name when the compound contains a cation which has different charges in different compounds. formula NaCl Al2S3 FeF3 Cu3(PO4)2 (NH4)2SO3 name sodium chloride aluminum sulfide iron(III) fluoride copper(II) phosphate ammonium sulfite How do we know when to specify the charge? ! By learning which cations can have more than one charge. The following ions will be used during this course: copper (1+, 2+); iron (2+, 3+); chromium (2+, 3+); tin (2+, 4+); and lead (2+, 4+). How can we know the charge of the cation in a particular formula? ! Remember when we write a neutral formula, the total number of positives must equal the total number of negatives. In formula writing the charges of the ions are used to determine the number of ions that make positives and negatives equal. Al3+ S2- 2(3+)=3(2-) Al2S3 How can we know the charge? ! In naming, the number of ions in the formula can be used to determine the unknown charge. In the example below, the formula tells us that there is one Sn ion and there are two O ions. O is in group 6A and therefore oxide ion’s charge is 2-. So the only unknown number is the charge of Sn. ! SnO2 1(?)=2(2-) Examples for you to try. How can we know the charge? ! Since the total number of positives must equal the total number of negatives, the charge of tin ion must be 4+. SnO2 1(?)=2(2-) 1(4+)=2(2-) tin(IV) oxide Remember: Number of cations(charge) = Number of anions(charge) FeF3 CrSO4 ! ! ! ! ! ! ! ! ! ! Cu3(PO4)2 Pb3N4 ! ! ! Check answers in the video.