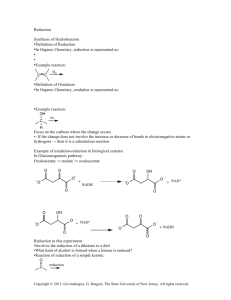
Benzil from Benzoin
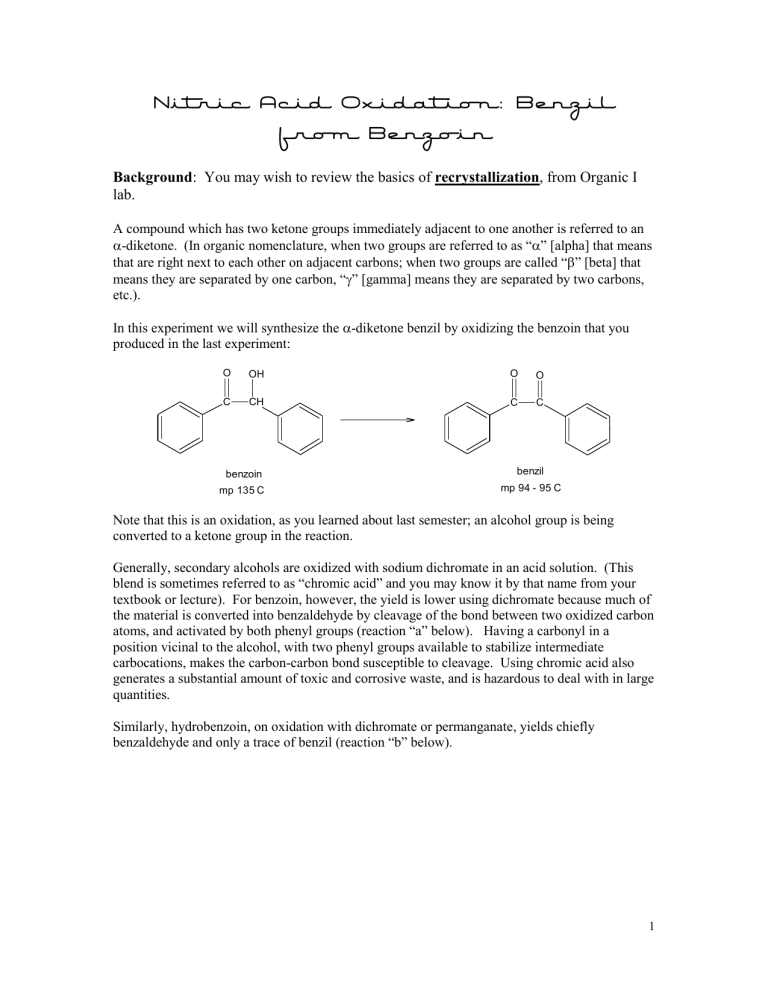
Nitric Acid Oxidation: Benzil from Benzoin
Background : You may wish to review the basics of recrystallization , from Organic I lab.
A compound which has two ketone groups immediately adjacent to one another is referred to an
-diketone. (In organic nomenclature, when two groups are referred to as “
” [alpha] that means that are right next to each other on adjacent carbons; when two groups are called “
” [beta] that means they are separated by one carbon, “ ” [gamma] means they are separated by two carbons, etc.).
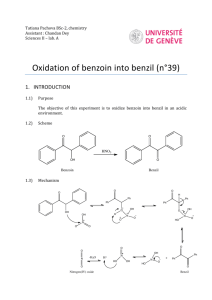
In this experiment we will synthesize the
-diketone benzil by oxidizing the benzoin that you produced in the last experiment:
O OH O O
C CH C C benzoin benzil mp 135 C mp 94 - 95 C
Note that this is an oxidation, as you learned about last semester; an alcohol group is being converted to a ketone group in the reaction.
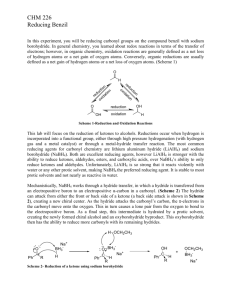
Generally, secondary alcohols are oxidized with sodium dichromate in an acid solution. (This blend is sometimes referred to as “chromic acid” and you may know it by that name from your textbook or lecture). For benzoin, however, the yield is lower using dichromate because much of the material is converted into benzaldehyde by cleavage of the bond between two oxidized carbon atoms, and activated by both phenyl groups (reaction “a” below). Having a carbonyl in a position vicinal to the alcohol, with two phenyl groups available to stabilize intermediate carbocations, makes the carbon-carbon bond susceptible to cleavage. Using chromic acid also generates a substantial amount of toxic and corrosive waste, and is hazardous to deal with in large quantities.
Similarly, hydrobenzoin, on oxidation with dichromate or permanganate, yields chiefly benzaldehyde and only a trace of benzil (reaction “b” below).
1
O
C
OH
CH
(a) benzoin
Cr
2
O
7
2-
H
2
SO
4
"chromic acid"
O
2 benzaldehyde
OH OH
CH CH oxid.
(b) hydrobenzoin
Benzoin can be oxidized to the
-diketone, benzil, very efficiently by nitric acid or by copper (II) sulfate in pyridine. To avoid cleavage of the molecule, the milder oxidizing agent nitric acid works well:
O OH O O
C CH C C
HNO
3
2
The mechanism of this is similar to chromic acid oxidation, but nitric acid is a milder reagent that will not cleave the C-C bond. Note that the conversion from the second intermediate to the third is an internal acid/base reaction, where a proton transfers from one oxygen to another:
O
O
OH
H O
O
-
N
+
O
H
O
+
O
-
N
+
O
-
OH
O
H
+
O
H
O
O
N OH
N
+
OH
H O
O
-
H O
O
-
The by-product, H
2
NO
3
-
is unstable; it loses water ( dehydrates ) to produce NO
2
gas.
----------------------------------------------------------------------------------------------------
Procedure: Nitric Acid Oxidation of Benzoin
Caution: Benzoin is an irritant to skin and mucus membranes!
Caution: Concentrated nitric acid is corrosive! It causes severe burns to the skin!
* note : the benzoin that you use in this experiment is your product from last week’s lab. You will need to complete the procedure from “The Benzoin Condensation” before beginning this experiment.
Set up a hot plate and begin a hot water bath (in a 400 or 600 mL beaker) IN THE HOOD.
If you have not already done so (last week), weigh the benzoin crystals from last week’s experiment. Record the mass in your notebook.
3
Caution!!!!
: this reaction will create extremely corrosive nitric acid fumes, and toxic nitrogen oxide fumes! Nitrogen oxides are also SEVERE irritants to the eyes, nose and mucus membranes.
Carry out this reaction in a hood ! Under no circumstances should you remove the flask from the hood while the reaction is going on! DO NOT remove the reaction mixture from the hood until the instructions indicate that you should do so .
Although nitrogen oxides are not absorbed through the skin, they will stain your skin yellow or brown -- you should wear gloves during this procedure.
In this reaction you will use about 4 grams of benzoin -- you should have plenty from last lab, but
MAKE CERTAIN THAT YOU DO NOT USE ALL THAT YOU HAVE -- you will need to save a small amount for the test at the end of this experiment. You only need to reserve a very small scoop for the last part; about 0.5 mg, enough to cover the tip of your spatula.
Weigh about 4 grams of the benzoin from last week’s experiment into a 125 mL Erlenmeyer flask. (If your yield from last week is less than 4 grams, your instructor can give you some extra for this procedure). IN THE HOOD , slowly add 14 mL of concentrated nitric acid. DO NOT
ADD THE HNO
3
ALL AT ONCE - the mixture may get hot and boil over; add the nitric acid in small portions until the entire 14 mL has been added. Place the flask in a boiling water bath for
10 minutes -- this also must be performed in the hood . It is best to perform this reaction in a
125 mL Erlenmeyer flask, to minimize the possibility of splattering.
The mixture will begin as a slurry mixture of solid and liquid; as it heats up to the temperature of the hot water, it should liquefy. You will also see the formation of dark orange or brown NO and
NO
2
fumes as the reaction proceeds.
After the 10 minute heating period, slowly add 75 mL of water to the reaction mixture, cool to room temperature, and swirl for a minute or two to coagulate the precipitated product; make sure that there are no nitrogen oxide fumes remaining in the flask. After this the reaction mixture may be removed from the hood . You can do the rest of the lab at your desk.
Note : At this point you may notice one or more globules of an oily substance in your flask. This is your product, but it may not solidify in acid solution.
SLOWLY add 25 mL of 6 M NaOH. Use litmus or pH paper to determine if the mixture is basic; if it is still acidic, keep adding NaOH, about 4 - 5 mL at a time, until the pH is basic.
(Depending on the exact concentrations of the NaOH and HNO
3
, this may take up to 40 or 50 mL of NaOH total). The product should begin to crystallize as you add the NaOH. Once the solution is basic, cool the mixture in an ice bath for a few minutes. Collect the yellow solid on a Büchner funnel and wash with water. Press the solid well on the filter to squeeze out the water. At this point you should check with your instructor to determine if the product needs to be recrystallized.
If you are going to perform the recrystallization - The crude product need not be dried or weighed but can be re-crystallized at once from ethanol.
If you are NOT going to perform the recrystallization - Remove the product from the filter paper and dry it in the oven for about 10 minutes. Weigh the crystals and proceed to the
“Test for the Presence of Unoxidized Benzoin.”
Recrystallization
Optional - ask your instructor if this step is required ! If not, skip to the next section “Test for the
Presence of Unoxidized Benzoin.” Heat about 10 - 15 mL of ethanol in a small Erlenmeyer flask on a hot plate (this can be done at your desk). Dissolve the product from the reaction above in a minimum amount of hot ethanol; remember that the mixture should be kept hot (on the hot plate)
4
until all of the solid has dissolved. One the solid is completely dissolved, add water dropwise until the solution becomes cloudy, and set aside to crystallize. You should place the mixture in an ice bath after crystals begin to form. Benzil is a fairly bright yellow solid; it looks similar to the benzoin but a brighter color.
Collect the benzil crystals by vacuum filtration. You may rinse the crystals and the container with distilled water if necessary. Allow the crystals to dry in the Büchner funnel, with air passing over them, for at least five minutes. Then transfer the crystals to a pre-weighed beaker or watch glass, and dry them in the oven for about 10 minutes. Then record the mass of your product.
Test for the Presence of Unoxidized Benzoin
This is a simple test to determine if your reaction is complete, and if your product is pure. This is a qualitative test, so it isn’t necessary to measure the quantities exactly; they can be approximated. In a test tube, dissolve about 0.5 mg (a small scoop, enough to cover the end of a spatula) of the benzil product in 0.5 mL of 95% ethanol or methanol and add one drop of
6 M sodium hydroxide. If unreacted benzoin is present, the solution soon acquires a dark brown or black color owing a complex of benzil with a product of auto-oxidation of benzoin. This reaction may also produce a muddy-looking precipitate. If no dark color develops in 1-3 minutes, and the sample remains a yellow or amber color, this indicates that the sample is free from benzoin (in other words, the oxidation of benzoin was complete).
Sometimes students are confused as to the results of this test because you have never seen what a
“positive” result looks like. You can observe a “positive” result if you add a small amount of benzoin; observe the color that develops. You can add a few more drops of NaOH and/or heat the mixture to speed up this appearance of the dark brown color after adding benzoin.
Cleaning Up
The aqueous filtrate is non-toxic, and should be neutral or slightly basic. Flush the waste down the drain, with plenty of water.
Report
Record the yield, color and melting point of the purified benzil. Depending on the procedure that you followed last week, you may need to measure the melting point of last week’s product, also.
Check with your instructor to see if this is required.
5
Benzil from Benzoin
Lab Report and Post-Lab
Name ____________________________________________________________
Data & Observations:
Starting materials:
Mass of Erlenmeyer Flask: _____________________ grams
Mass of Erlenmeyer Flask + Benzoin:
Mass of Benzoin:
Product:
Mass of container:
_____________________ grams
_____________________ grams
_____________________ grams
Mass of container + Benzil: _____________________ grams
Mass of Benzil (product): _____________________ grams
Melting point of product (if determined): _____________________
Post-Lab Questions ( please answer on a separate sheet ):
C
1. Report the yield and % yield of benzil; be certain to show your calculations for both theoretical yield and % yield.
2. In the
1
H-NMR spectrum for benzoin, how many signals would you predict to see
(assuming the aromatic hydrogens will display “accidental” equivalence)?
3. In the
1
H-NMR spectrum for benzil, how many signals would you predict to see
(assuming the aromatic hydrogens will display “accidental” equivalence)?
4. In the
13
C-NMR spectrum for benzoin, how many signals would you expect to see?
Which signal would be most de-shielded in this spectrum?
5. In the
13
C-NMR spectrum for benzil, how many signals would you expect to see?
Which signal would be most de-shielded in this spectrum?
6. What would be the principle peaks observed in the IR spectrum for benzoin?
7. What would be the principle peaks observed in the IR spectrum for benzil?
8. Summarize and explain your observations from the “Test for the presence of unoxidized benzoin”:
6
Pre-Lab Questions Benzil from Benzoin
Name ____________________________________________________________
1. Calculate the theoretical yield of benzil in grams , starting with 4.00 grams of benzoin.
Show your work ! (NOTE: You may wish to make a copy of this calculation before submitting the Pre-Lab, as you will be doing a very similar calculation in the lab report !)
7