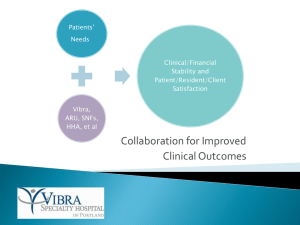
November 2013
advertisement

November 2013 In this issue Page Administration Misrouted Protected Health Information (PHI) Important reminder: 2013 National Uniform Billing Committee (NUBC) UB-04 Code Changes Attention psychiatrists and psychiatric offices: Corrections to behavioral health claims Make sure your transplant global case rate claims are complete and correct Americold Logistics, LLC returns to Anthem National Accounts BCBSA announces agreement with Verisk Health for medical record requests ICD-10 Check-up: How am I doing? Get it right the first time – Receive payments accurately, predictably and reliably Clinical practice and preventive health guidelines available on the web Health care reform updates (including Health Insurance Exchange) 2 2 4 4 5 5 6 6 7 7 Products and programs 2014 FEP Benefit information available online Important 2014 diabetic supply coverage changes for DME providers AIM sleep disorder guideline updates and program change Quality-In-Sights® Pay for Performance survey is due January 15, 2014! HEDIS® 2013 results Habit Heroes delivers message of good health Case Management: Better health for patients who need care the most NurseLine offers 24/7 access to nurses Future Moms promotes healthy pregnancies and healthy babies MyHealth Advantage works with you to help your patients 7 7 9 9 10 14 15 15 16 17 E-business ProviderAccess is going away Nov. 8 th Interactive Care Reviewer (ICR) now accepts inpatient requests 18 19 bcbsga.com State Health Benefit Plan Important information about the State Health Benefit Plan 20 Important phone numbers Senior business and Medicare Advantage Please ensure referred physicians are in the Medicare Advantage Network 1 of 39 21 Senior business and Medicare Advantage continued Physicians to receive home test kits results for Medicare Advantage Members Record exact adult BMI number, not range Update: Avoidable Medicare Advantage readmissions may lead to administrative claim denials Medicare reports on osteoporosis management after bone fracture Medicare Advantage Part D formulary changing for 2014 2014 Medicare Advantage coverage changes for diabetic supplies American Diabetes Association offers medication guidelines for patients with diabetes/hypertension Speaking the Language of ICD-10 - Part 2 21 21 22 22 23 23 24 24 Pharmacy Pharmacy information available on bcbsga.com Coverage for compound drugs 26 26 Policy updates Always Bundled Policy: Certain bundled services ineligible for separate reimbursement Medical Policy and Clinical Guideline updates 26 27 Administration Misrouted Protected Health Information (PHI) Providers and Facilities are required to review all member information received from BCBSGa to ensure no misrouted PHI is included. Misrouted PHI includes information about members that a Provider or Facility is not currently treating. PHI can be misrouted to Providers and Facilities by mail, fax, or e-mail. Providers and Facilities are required to immediately destroy any misrouted PHI or safeguard the PHI for as long as it is retained. In no event are Providers or Facilities permitted to misuse or re-disclose misrouted PHI. If Providers or Facilities cannot destroy or safeguard misrouted PHI, Providers and Facilities must contact Provider Services at 800-428-4446 to report receipt of misrouted PHI. Important reminder: 2013 National Uniform Billing Committee (NUBC) UB-04 Code Changes The following changes have been made by the National Uniform Billing Committee (NUBC) and are outlin ed below: New Patient (Discharge) Status Codes Type of Bills (TOB) 32X and 34X: Description Change Type of Bill 33X: Code Eliminated Type of Bills 084X and 089X: Revised New Revenue Code: 0953 Source of Admission: Required on all Types of Bills except 014X November 2013 2 of 39 New Patient Discharge Status Codes (FL-17) approved by NUBC effective October 1, 2013 are as follows: NOTE: Patient Discharge status codes are required by NUBC on both the inpatient and outpatient claims. Value Description 69 81 Discharge transferred to a designated disaster alternate care Discharged to Home or Self Care with a Planned Acute Care Hospital Inpatient Readmission Discharged/Transferred to a Short Term General Hospital for Inpatient Care with a Planned Acute Care Hospital Inpatient Readmission Discharged/Transferred to a SNF with Medicare Certification with a Planned Acute Care Hospital Inpatient Readmission Discharged/Transferred to Facility that Provides Custodial or Supportive Care with a Planned Acute Care Hospital Inpatient Readmission Discharged/Transferred to a Designated Cancer Center or Children’s Hospital with a Planned Acute Care Hospital Inpatient Readmission Discharged/Transferred to Home Under Care of Organized Home Health Organization with Planned Acute Care Hospital Inpatient Readmission Discharged/Transferred to Court/Law Enforcement with a Planned Acute Care Hospital Inpatient Readmission Discharged/Transferred to a Federal Health Care Facility with a Planned Acute Care Hospital Inpatient Readmission Discharged/Transferred to a Hospital-based Medicare Approved Swing Bed with a Planned Acute Care Hospital Inpatient Readmission Discharged/Transferred to an IRF including Rehabilitation Distinct Part of a Hospital with a Planned Acute Care Hospital Inpatient Readmission Discharged/Transferred to a Medicare Certified Long Term Care Hospital (LTCH) with a Planned Acute Care Hospital Inpatient Readmission Discharged/Transferred to Nursing Facility Certified by Medicaid but not Certi fied by Medicare with Planned Acute Care Hosp IP Readmission Discharged/Transferred to Psychiatric Hospital or Psychiatric Distinct Part of a Hospital with a Planned Acute Care Hosp IP Readmission Discharged/Transferred To a Critical Access Hospita l (CAH) with a Planned Acute Care Hospital Inpatient Readmission Discharged/Transferred to Another Type of Health Care Institution not Defined in this Code List with a Planned Acute Care Hosp IP Readmission 82 83 84 85 86 87 88 89 90 91 92 93 94 95 Type of Bill (FL-4) Description Changes: 032X Description change - Home Health Services under a Plan of Treatment (eff 10/01/13) 034X Description change - Home Health Services not under a Plan of Treatment (eff 10/01/13) Type of Bill (FL-4) Eliminated: 033X Code Eliminated - No longer a valid code as of 10/01/13 November 2013 3 of 39 Type of Bill changes (FL-4) from Inpatient / Outpatient to Outpatient ONLY: 084X Change from Inpatient / Outpatient to Outpatient only service designation - Specialty Facility Free Standing Birthing Center (eff 07/01/12) 089X Change from Inpatient / Outpatient to Outpatient only service designation - Specialty Facility Other (eff 07/01/13) New Revenue Code (FL-42): 0953 Chemical Dependency effective for claims received on or after 10/01/2013 Source of Admission (Point of Origin) (FL-15) required on all claims (except 014x): Effective 10/01/2013, the Blue Cross Blue Shield Association (BCBSA) is requiring Source of Admission/Point of Origin codes for all TOB's (except 014X Lab services). This BCBSA ALL modification is needed by Home Plans for pricing non-Par claim encounters and is in alignment with the NUBC changes. Please make sure all billing staffs are aware of these changes. Changes affect Commercial, FEP and Medicare Advantage products. Additional information regarding the Discharge status codes may be communicated by CMS in the future. Attention psychiatrists and psychiatric offices: Corrections to behavioral health claims As you know, the American Medical Association adopted new CPT codes for behavioral health services effective January 1, 2013. We are writing to let you know about certain technical difficulties BCBSGa has had in implementing those codes. Specifically, we have experienced claims processing system errors as a result of the new codes that might have affected the payments you received for services provided since January 1, 2013, and also might have resulted in incorrect and/or multiple deductibles and co-payments for your patients. This problem is being addressed with the highest priority. In order to ensure that you have been properly reimbursed and that your patients have not paid more than their plans require, we also are reviewing claims that may have been affected by these system errors. Where we identify claims that were processed incorrectly, we will re-process the claim and correct the co-payment due from the member, and you will receive a new remit with the correct amounts attributed to your patients. You do not need to resubmit claims to have them reprocessed; we will do that automatically. However, if you have any questions or concerns about whether a claim was processed correctly, please do not hesitate to contact Provider Services at 800 -428-4446. We apologize for any inconvenience and confusion these system errors may have caused. BCBSGa values your commitment to our members, and we want to assure you that we are committed to making sure you are properly reimbursed for the services you provide. Make sure your transplant global case rate claims are complete and correct When submitting a transplant global case rate claim it is imperative to include a cover sheet or an Attachment H, whichever is applicable, that clearly reflects all the claim information included in the global case rate package. Prior to sending a global case rate package, review the claim information included in the package to make sure that it matches what is indicated on November 2013 4 of 39 the cover sheet or Attachment H. All claims pertaining to the transplant must be included in the global case rate packet. Individual transplant claims submitted separately by an individual physician may be denied because that claim should be included in the global case rate claim. When a global case rate package is received by BCBSGa, we review the claim to ensure that the cover sheet or Attachment H accurately reflects the claims included in the package. If the claims included do not match the information indicated on the cover sheet or Attachment H, BCBSGa will require more information and claim payment will be delayed. Individual providers that are unclear of transplant global case rate claim guidelines should contact the facility performing the transplant in order for everyone to receive timely reimbursement. If you have any questions about this information please contact your Provider Repr esentative. Americold Logistics, LLC returns to Anthem National Accounts Beginning January 1, 2014, Americold Logistics, LLC will be administered by the Richmond Service Center in Virginia. Americold Logistics associates and their enrolled family members who live in Georgia will be covered under the Blue Open Access PPO network and will receive new member ID cards with the alpha prefix LQZ. Those living outside of the Blue Open Access POS network area will use the BlueCard PPO network and will receive new member ID cards with the alpha prefix LGO. Please remember to verify the member’s ID card on each visit to ensure your claims are submitted with the correct member ID for that particular date of service. Electronic claim submission is the most efficient way to submit your claims; however, if you submit a hard copy claim please refer to the back of the members ID card for the appropriate claim submission address and customer service number. For questions or additional information, call the Americold Log istics dedicated customer service center at 877-344-2942. BCBSA announces agreement with Verisk Health for medical record requests The Blue Cross and Blue Shield Association (BCBSA) has contracted with vendor Verisk Health to obtain out -of-area medical records for all Blue Plans. (Verisk Health helps employers, providers and payers mitigate risk, reduce health care costs and improve patient outcomes.) BCBSGa supports the association’s agreement that allows Blue Plans – if appropriate – to request medical records from a provider who is out of the Plan’s service area. These medical record requests support HEDIS reporting, commercial risk adjustment and Medicare risk adjustment. It is important to note that medical record requests for the purpose of cla ims processing will remain the same. Beginning October 2013, providers may receive letters or calls from Verisk Health with record requests for patients who are members of a Blue Plan outside of our service area. Please be assured that all medical information will be used and maintained in a confidential manner in accordance with privacy regulations specified by the Health Insurance Portability and Accountability Act (HIPAA) and BCBSGa corporate policies. As a reminder, you do not need the member’s permission to release medical records associated with this request. November 2013 5 of 39 As in the past, the time frame for obtaining and reporting HEDIS and risk adjustment information is limited. The return of requested medical records is usually five business days. We appre ciate your cooperation and time in working with us to promptly submit the requested information. ICD-10 Check-up: How am I doing? The end of the year is a great time to look back and review the status of important projects and activities. The implementation plan for your practice’s transition to ICD-10 is one of those long term efforts where periodic check -ups can help to make sure you are still on target to be ready by October 1, 2014. Below is a list of some of the planning activities that your practice should have completed during the last year. Processes/Workflows Changes – You should have identified which internal processes and workflows will be affected by using the new code set, how each workflow is affected and have a plan to address the change s that need to occur to incorporate ICD-10. Systems Changes – Assessment of all systems used by your practice should be completed. You should have a comprehensive list of all necessary system changes, upgrades and/or other adjustments, the cost of these c hanges, the amount of time it will take to complete these changes and the timeline for implementation. External Partners – You should have a clear picture of how each of your vendors, clearinghouses and/or billing services plan to handle the transition to ICD-10 and how their plan will affect your practice. Documentation Requirements –You should have assessed a sample of your practice’s patient records to determine if the clinical documentation is complete and detailed enough to properly code claims using I CD-10 diagnosis codes. With the new level of specificity of each code, having the right documentation available for your medical coders will lessen the potential for decreased productivity associated with using the new code set. Training – You should have a comprehensive list of the education and training needs for your staff members. The list should detail the type of training needed (coding, systems, etc.), who will receive the training and timeline for the training to occur. Training costs should also be determined and budgeted as appropriate in future fiscal planning. As you move into the active phases of your implementation plan for ICD-10, having this knowledge as your foundation will be the key to a smooth transition to ICD-10. Get it right the first time – Receive payments accurately, predictably and reliably While the coordination of benefits process works well most of the time, difficulties associated with the process — from paperwork to inaccurate payments to claims appeals — make the healthcare system cumbersome and have long burdened the healthcare industry. Typically, coordination of benefits issues stem from confusion over a patient’s insurance status, particularly for individual s who have lost or changed jobs or have multiple sources of coverage. Recognizing that these difficulties have cost the healthcare system millions, BCBSGa is working closely with other health plans and CAQH® to improve the accuracy of coordination of benefits processes for providers and patients. COB Smart™, a CAQH® Solution, is a registry of coverage information that will correctly identify which patients have benefits that should be coordinated in order for corresponding claims to be processed correctly the first time. Starting November 3, 2013, each week, BCBSGa and other CAQH participating health plans and clearinghouses will supply coverage information to the registry, where it will be compared with information from other participating health plans to identify individuals with more than one form of coverage. Standard primacy rules are then applied to determine the correct order of benefits. Starting in 2014, providers can access this complete and accurate coordination of benefits information through processes that integrate with most existing workflows. November 2013 6 of 39 The CAQH COB Smart registry will help increase payment accuracy and timeliness, reduce paperwork and improve cash flow for all providers. This solution will save the system money by reducing administrative resources and hassle when determining coverage. To learn more about COB Smart, and BCBSGa’s involvement, please review the Frequently Asked Questions on the Answers@BCBSGa page of our provider website, bcbsga.com. Clinical practice and preventive health guidelines available on the web As part of our commitment to provide you with the latest clinical information and educational materials, we have adopted nationally recognized medical, behavioral health, and preventive health guidelines, which are available to providers on our website. The guidelines, which are used for our Quality programs, are based on reasonable, medical evidence, and are reviewed for content accuracy, current primary sources, the newest technological advance s and recent medical research. All guidelines are reviewed annually, and updated as needed. The current guidelines are available on the Health & Wellness page of our provider website, bcbsga.com. Health care reform updates Health care reform updates and notifications and Health Insurance Exchange information is posted as they become available on the communications page of bcbsga.com. Articles titled “Important information about provider referrals for plans purchased on and off the Exchange ” and “Many members will have new health plans in 2014” have been posted to the Health Insurance Exchange page of bcbsga.com. Products and programs 2014 FEP Benefit information available online To view the 2014 benefits and changes for the Blue Cross Blue Shield Service Benefit Plan, also known as the Federal Employee Program (FEP), go to bcbsga.com/fep and select a Coverage Options in the tool bar. Here you will find the 2014 Service Benefit Plan Brochure and Plan Benefit Summary information for year 2014. For questions please contact FEP Customer Service at 800-282-2473. Important 2014 diabetic supply coverage changes for Durable Medical Equipment (DME) providers Effective January 1, 2014, all of our Individual Medicare Advantage Plans will only cover LifeScan, Inc., OneTouch ® or Roche Diagnostics, ACCU-CHEK ® diabetic glucometers and blood test strips. This benefit change is meant to help control out-of-pocket expenses while not compromising on quality. Covered glucometers and blood test strips in 2014: LifeScan, Inc., OneTouch ® Roche Diagnostics, ACCU-CHEK ® A limit of 100 blood test strips per month November 2013 7 of 39 Other blood glucometers or blood test strip brands or more than 100 test strips per month are not covered unless the member’s doctor or other treating provider tells us that another brand or a larger quantity is medically necessary for their treatment. What will you need to do? If your customers are currently using OneTouch ® or ACCU-CHEK ® blood test strips or glucometer products, no action is required on your part. If your customers are not using OneTouch ® or ACCU-CHEK ® blood test strips and glucometer products, you will need to have the customer get new prescriptions from their doctor for their supplies by January 1 st in order for these claims to be covered. If the customer’s doctor says it is medically necessary for them to continue using a different brand of blood test strips or glucometer and/or more than 100 blood test strips per month, their doctor will need to call the provider service number listed on the back of the member’s card. If the customer has questions, direct the customer to call the health plan’s customer service number found on the back of their ID card. Grace Period: To allow you time to transition these customers over to LifeScan, Inc., OneTouch ® or Roche Diagnostics, ACCUCHEK ® blood test strips and glucometers we will continue to cover the current brands for up to two fills during the first 90 days of the year. After the grace period has expired, if a network provider bills for non-covered brands or exceeds the quantity limit without having an exception on file, the DME provider will be liable for the charges, not the member. As always, we reserve the right to conduct random audits to ensure compliance with the plan’s benefit administration. Plans that are included in this coverage change include: CONTRACT # Plan Benefit Package (PBP) PLAN NAME H5422 002 BlueValue Secure (HMO) H5422 006 BlueValue Basic (HMO) H9947 001 Medicare Preferred Core (PPO) PLAN TYPE GA-HMO GA-HMO GA-LPPO If you are in doubt as to whether or not your customer is one of our Individual Medicare Advantage Prescription Drug (MAPD) versus an Employer or Union Sponsored plan, please have them check the front of the ID card which will show the contract and Plan Benefit Package (PBP) number (example: H1234-001). Note: If the PBP (the last three digits of the contract-PBP number) is in the 800 series, that member is in an Employer or Union Sponsored plan and these changes do not apply to their plans. Please contact the plan’s Provider Service Department listed on the back of the member’s ID card if you have any questions about these coverage changes. The benefit information provided is a brief summary, not a complete description of benefits. For more information, contact the plan. Limitations, copayments, and restrictions may apply. Benefits, formulary, pharmacy network, provider network, premium and/or co-payments/co-insurance may change on January 1 of each year. For more information about the exception process or the appeals policy, pleas e see the plan’s Provider Manual located on the Answers@BCBSGa page of our provider website, bcbsga.com. November 2013 8 of 39 AIM sleep disorder guideline updates and program change Effective for dates of service on or after January 1, 2014, the following AIM Specialty Health SM (AIM) Sleep Disorder Management Diagnosis and Treatment Guidelines will be revised as follows: A new guideline, Utilization of Multiple Sleep Latency Testing (MSLT) and Maintenance of Wakefulness Testing (MWT), has been added for the diagnoses of narcolepsy and hypersomnia. This guideline replaces the previous BCBSGa clinical guideline CG-MED-43, Multiple Sleep Latency Testing (MSLT) and Maintenance of Wakefulness Testing (MWT). The following clarifications have been made: – The terms “respiratory disturbance index” (RDI) and “apnea hypopnea index” (AHI) may be used interchangeably. – When an attempt has been made to use auto-titrating positive airway pressure (APAP) to titrate continuous positive airway pressure (CPAP) or bi-level positive airway pressure (BPAP) and that attempt has been unsuccessful, in-lab titrations may be performed. – The list of contraindications to home sleep testing has been expanded. Individuals meeting criteria for a sleep study that have any of these contraindications should have their studies performed at a facility. – The oral appliances guideline has been clarified to indicate that prefabricated oral appliances are not considered to be clinically appropriate under any circumstances. The current and revised guidelines can be accessed at aimspecialtyhealth.com or on the Medical Policy, Clinical UM Guidelines, and Pre-Cert Requirements section of our provider website, bcbsga.com. Please note that these changes are effective January 1, 2014. Please note: With the adoption of the new guideline, “Multiple Sleep Latency Testing (MSLT) and Maintenance of Wakefulness Testing (MWT),” as part of our sleep program managed by AIM, effective January 1, 2014, MSLT and MWT (CPT code 95805) will now be reviewed by AIM, rather than BCBSGa, along with polysomnography and home sleep testing. This requirement is effective for members of our local and individual health plans who participate in the sleep management program managed by AIM. Quality-In-Sights® Pay for Performance survey is due January 15, 2014! It is once again time to submit your Quality-In-Sights® Pay for Performance Practice Survey. If you have not already done so, please remember to submit your Provider Survey for the BCBSGa Quality In -Sights Pay for Performance Program by January 15, 2014. The 2013 Measurement Year Practice Survey can be accessed through our secure provider portal, ProviderAccess or by clicking here. To access the survey through ProviderAccess, the member of your staff who is the Account Administrator for your ProviderAccess account will need to log in. Once logged into ProviderAccess, you will see the "Rewards and Recognition" banner. Click on the banner, acknowledge that you are not a third party billing entity, and you will be taken to the POIT home page. On the POIT home page, you will find the survey on the "Prog rams" tab; click "Start Survey" in the box labeled "Technical Survey." Information on completing the survey for the Quality-In-Sights Primary Care Incentive Program can also be found on our public provider website. You can complete the survey in its electronic format by clicking here. You will need to save the survey to your computer, fill it out and then email it to the email address provided in the survey document. The survey is necessary to satisfy some of the measures of the Program. Only one Practice Survey is required for all practice locations under a single tax id. Surveys must be completed and submitted no later than January 15, 2014 to November 2013 9 of 39 be considered in your 2013 Quality-In-Sights score. Surveys can be submitted through the ProviderAccess POIT link or via email. Surveys cannot be mailed or faxed. If you have any questions regarding this information, please send us an email at prrprogramsga@bcbsga.com, call program support at 888-650-5740 or contact your Network Representative directly. HEDIS® 2013 results Thank you for participating in the annual Healthcare Effectiveness Data and Information Set (HEDIS) data collection for 2013. You play a central role in promoting the health of our members. One way to improve care is to document ser vices in a consistent way. This makes it easy for you to track care provided and see what additional care is needed to meet the recommended timeframes. Consistent documentation will also help improve HEDIS scores, both by improving care itself and by improving our ability to report validated data. Further information regarding HEDIS documentation guidelines will soon be available on the Quality page under the Health & Wellness section of our provider website, bcbsga.com. There you will soon find reference documents entitled “HEDIS Timeline,” “HEDIS 101 for Providers” and “HEDIS Documentation Guidelines”. The table below shows comparison of some of our key measure rates to the NCQA Commercial HMO/POS Measures HEDIS 2013 Rate (Percent) Effectiveness of Care – Prevention and Screening Adult BMI Assessment 69.34 Breast Cancer Screening 67.07 Cervical Cancer Screening 74.40 Childhood Immunization Status - DTAP 89.05 Childhood Immunization Status - MMR 89.78 Childhood Immunization Status – HEP B 92.94 Childhood Immunization Status - PCV 88.08 Childhood Immunization Status – HEP A 42.58 Childhood Immunization Status - ROTAVIRUS 77.86 Childhood Immunization Status - INFLUENZA 54.01 Colorectal Cancer Screening 61.73 Immunizations for Adolescents - MENINGITIS 67.50 Immunizations for Adolescents – TDAP/TD 77.50 Weight Assessment and Counseling – BMI TOTAL 44.53 Weight Assessment and Counseling – Physical Activity- TOTAL 45.01 Access/Availability of Care Children & Adolescents’ Access to PCP (25 mos-6yrs) 92.30 Children & Adolescents’ Access to PCP (12-19 yrs) 85.41 Effectiveness of Care – Respiratory Conditions Appropriate Testing for Children w/ Pharyngitis 82.50 Appropriate Treatment Children w/ URI 82.37 Spirometry Testing for COPD 46.68 November 2013 Quality Compass ® National Averages. 2013 National Comparison to Average National Average (Percent) 66.11 70.27 75.50 87.18 91.82 89.17 86.72 65.50 76.67 63.33 63.26 65.99 79.24 51.57 50.37 91.64 89.68 80.20 84.04 43.52 10 of 39 Effectiveness of Care - Cardiovascular Cholesterol Management – LDL-C Control <100 Persistence of Beta-Blocker Treatment after AMI Effectiveness of Care - Diabetes Comprehensive Diabetes Care – HbA1c Testing Comprehensive Diabetes Care – Poor HbA1c Control (>9)* Comprehensive Diabetes Care – Eye Exams Comprehensive Diabetes Care – LDL-C Screening Comprehensive Diabetes Care – LDL-C Controlled (LDL-C<100 mg/dL) Comprehensive Diabetes Care – Blood Pressure Control <140/80 Comprehensive Diabetes Care – Blood Pressure Control <140/90 Effectiveness of Care - Musculoskeletal Use of Imaging Studies for Low Back Pain Effectiveness of Care – Behavioral Health Antidepressant Medication Mgmt – Acute Antidepressant Medication Mgmt – Continuation FU Care Children’s ADHD Medication – Initiation FU Care Children’s ADHD Medication - Continuation Utilization & Relative Resource Use Well-Child Visits in the first 15 Months of Life (6+ visits) Adolescents Well-Care Visits 65.36 74.45 59.87 83.86 92.88 22.45 45.80 88.50 49.45 90.09 28.47 56.82 85.42 48.42 40.33 44.34 67.52 66.48 67.40 75.26 64.02 47.44 35.10 40.82 69.15 53.58 38.64 45.71 76.04 35.01 78.19 43.26 *lower rate is better Commercial PPO Measures Effectiveness of Care – Prevention and Screening Adult BMI Assessment Breast Cancer Screening Cervical Cancer Screening Childhood Immunization Status – DTAP Childhood Immunization Status – IPV Childhood Immunization Status – MMR Childhood Immunization Status – HIB Childhood Immunization Status – HEP B Childhood Immunization Status – VZV Childhood Immunization Status – PCV Childhood Immunization Status – HEP A Childhood Immunization Status – ROTAVIRUS Childhood Immunization Status – INFLUENZA Colorectal Cancer Screening Immunizations for Adolescents – MENINGITIS November 2013 HEDIS 2013 Rate (Percent) 2.69 64.73 70.84 63.01 68.98 81.68 72.03 46.46 82.43 63.06 78.17 56.17 50.63 47.75 49.03 2013 National Average (Percent) Above or below National Average 35.17 66.52 73.61 79.98 86.26 88.32 88.29 77.32 88.04 78.94 61.12 69.77 59.84 55.77 57.05 11 of 39 Immunizations for Adolescents – TDAP/TD Weight Assessment and Counseling – BMI TOTAL Weight Assessment and Counseling – Nutrition Counseling TOTAL Weight Assessment and Counseling – Physical Activity TOTAL Access/Availability of Care Children’s & Adolescents’ Access to PCP (25 mos-6 yrs) Children’s & Adolescents’ Access to PCP (7-11 yrs) Children’s & Adolescents’ Access to PCP (12-19 yrs) Effectiveness of Care – Respiratory Conditions Antibiotic Treatment Adults w/ Acute Bronchitis Appropriate Testing for Children w/ Pharyngitis Appropriate Treatment Children w/ URI Utilization & Relative Resource Use Well-Child Visits in the first 15 Months of Life (0 visits) Well-Child Visits in the first 15 Months of Life (1 visit) Well-Child Visits in the first 15 Months of Life (3 visits) Well-Child Visits in the first 15 Months of Life (6+visits) Adolescent Well-Care Visits Effectiveness of Care - Cardiovascular Cholesterol Management – LDL-C Control <100 Persistence of Beta-Blocker Treatment after AMI Effectiveness of Care - Diabetes Comprehensive Diabetes Care – HbA1c Testing Comprehensive Diabetes Care – Poor HbA1c Control (>9)* Comprehensive Diabetes Care – Eye Exams Comprehensive Diabetes Care – LDL-C Screening Comprehensive Diabetes Care – LDL-C Controlled (LDL-C<100 mg/dL) Comprehensive Diabetes Care – Medical attention for nephropathy Effectiveness of Care – Behavioral Health Antidepressant Medication Mgmt – Acute Antidepressant Medication Mgmt – Continuation FU Care Children’s ADHD Medication – Initiation FU Care Children’s ADHD Medication - Continuation 58.23 2.58 0.95 69.92 31.19 35.38 0.52 32.57 82.92 84.20 79.25 90.08 90.51 87.60 29.01 77.61 78.12 21.37 78.94 82.33 9.52 2.61 3.54 63.77 29.47 2.88 1.31 2.11 76.36 40.15 79.96 78.77 49.66 79.47 84.03 88.36 27.30 77.85 8.87 87.17 35.25 48.80 81.68 41.65 68.35 78.59 66.12 48.78 34.95 41.95 68.91 53.40 38.11 44.94 *lower is better In Georgia many scores for Commercial HMO showed either slight improvement or exceeded the National Average, not both. Some of the largest increases over last year include Persistence of Beta -Blocker Treatment after AMI with 6.62%, Antidepressant Medication Mgmt-Acute with 7.31%, Antidepressant Medication Mgmt-Continuation 5.46%, FU Care Children’s ADHD Medication-Initiation 2.82% and FU Care Children’s ADHD Medication-Continuation 3.82%. Each of these remains below the National Average. November 2013 12 of 39 The Commercial PPO shows double digit rate increases for many of the immunization measures including DTAP 18.43%, IPV 17.73%, MMR 5.85%, HIB, 14.76%, HEP B 27.82%, VZV 5.96%, PCV 16.93%, HEP A 47.88%, Rotavirus 16.21%, Influenza 9.82% and Combo 2 increased 26.24%. Colorectal Cancer Screening increased 6.41%, Persistence of Beta -Blocker Treatment after AMI increased 15.01% and Comprehensive Diabetes Care -Nephropathy increased 10.85%. While much improved only Hep A exceeds the National Average. Other increases a re in the less than 5% range and do not meet the Average. Overall, the Commercial HMO rates fell 5% or less from last year. There are opportunities for improvement for the measures with the most significantly decreased rates including: Cervical Cancer Screening which fell 2.33% and both IMA measures fell, TDAP 2.19% and Meningococcal 2.55% while Meningococcal did exceed the National Average. All Medication Management measures fell this year with declines ranging from 3.36% to11.35% for Digoxin. Appropriate Testing for Children with Pharyngitis fell 1.96% but exceeded the National Average. This year the Commercial PPO plan had the greatest number of decreased rates, although some were above the National Average. The most significant declines were the FU after Hospitalization for Mental Illness -30 days 24.36% and 7 days 16.54%. The Comprehensive Diabetes Care-Eye Exams fell 7%, Children’s & Adolescents’ Access to PCP 12 -24 months fell 7.53% and 25 months - 6yrs fell 4.08%. November 2013 13 of 39 Each year our goal is to improve our process for requesting and obtaining medical records for our HEDIS project, and to demonstrate the exceptional care that you have provided to our members. In an effort to improve our scores, you and your office staff can help facilitate the HEDIS process improvement by: Responding to our requests for medical records within five days Providing the appropriate care within the designated timeframes Accurately coding all claims Documenting all care in the patient’s medical record Again, we thank you and your staff for demonstrating teamwork and partnership as we work together to improve the health of our members and your patients. We look forward to working with you next HEDIS season. The source for data contained in this publication is Quality Compass® 2013 and is used with the permission of the National Committee for Quality Assurance (NCQA).Quality Compass 2013 includes certain CAHPS data. Any data display, analysis, interpretation, or conclusion based on these data is solely that of the authors, and NCQA specifically disclaims responsibility for any such display, analysis, interpretation, or conclusion. Quality Compass is a registered trademark of NCQA. HEDIS® is a registered trademark of the National Committee for Quality Assurance (NCQA) Habit Heroes delivers message of good health Through a joint alliance with Walt Disney World® Resort, BCBSGa has launched Habit Heroes™, an interactive, multimedia experience designed to bring the message of good health to people of all ages. An interactive comic adventure, Habit Heroes™ aims to teach families about building healthy habits. The program includes an exhibit at INNOVENTIONS at Epcot® at Walt Disney World® Resort, an informational website and an innovative mobile application. The free mobile application features the Habit Heroes comic characters and fun tools for tracking and building “hero power,” and is available for download by anyone at habitheroes.com, iTunes or Google Play. Supporting Habit Heroes™ is one way BCBSGa is working to reverse the trends of unhealthy lifestyle choices, which can lead to obesity and rising health care costs. A recent Centers for Disease Control study 1 showed staggering statistics, revealing that 17 percent of U.S. citizens ages 2–19 are obese, and nearly 80 percent of children who were overweight between ages 10 and 15 were obese by age 25. A limited number of comic books featuring the Habit Heroes™ characters are available in both English and Spanish free of charge. If you are interested in sharing these with your patients, please contact your local Network Relations consultant. November 2013 14 of 39 Case Management: Better health for patients who need care the most Our Case Management program helps members who are at risk for greater illness and cost, and would benefit from outreach and support. Our goal is to offer information and guidance to optimize members’ health, from the very sick to those returning to full health. We offer Case Management to members who are currently going through major medical care. We also reach out to members who are likely to need a lot of health care services in the near future. Our goals are: To help you by supporting your plan of care for your patients. To help your patients have better health, which could lower their cost of care. Who qualifies for the program? Case Management uses more than 500 health markers to find members who may be at highest risk for serious conditions. We also reach out to members who currently have serious health problems. These are members who are going through major procedures and treatments and may need extra help and follow-up care. Some typical conditions for which members often need significant care: Multiple sclerosis Severe heart problems Major stomach diseases Severe neurological issues Any major hospitalization from a diagnosis or injury How the program works We call members who qualify for the program and tell them how we can help. When they join the program, our nurse coaches work closely with them to support your treatment plan. The nurses answer questions about diagnosis and drugs, and set goals for better health. Members who have recently left the hospital get support on discharge planning, home health care, follow-up appointments and community resources. We focus mainly on spotting and resolving gaps in care. Our outreach for enrollment includes live and automated telephone calls and letters to members. Case Management gets a thumbs-up* More than 86% of members were “satisfied” or “very satisfied” with Cas e Management. 93% of members thought our program was “extremely valuable” or “very valuable.” *2012 Member Satisfaction Survey NurseLine offers 24/7 access to nurses Our 24/7 NurseLine program gives our members access to trained registered nurses any time of the day or night. Members can get help deciding what kind of care they need. NurseLine is a great resource for members, especially after office hours – and even on weekends. 24/7 NurseLine doesn’t replace medical care or handle emergencies. During the call, if the nurse believes that the member needs emergency care, the nurse starts a three-way 911 call. The nurse stays on the call until emergency help comes. November 2013 15 of 39 How 24/7 NurseLine serves members: 24/7 NurseLine records and tracks each call along with data from our programs. For instance, if a member is enrolled in our diabetes program, the nurse can access the member’s records. Members can get help based on their health history and receive resources to drive their care. The NurseLine staff is skilled and dedicated. Our nurses average 19 years of nursing experience. They also have quick access to doctor support on all shifts. Each call center has a medical director who provides clinical oversight and advice to our nurses. We use a huge database with about 5,000 topics. They cover signs, medical tests, drugs, wellness and general health problems. Our audio library has more than 400 topics in both English and Spanish. We have nurses who speak Spanish and a language line for interpreters for most languages. Members whose benefit plans include 24/7 NurseLine may have the toll-free number on the back of their member ID card. 24/7 NurseLine by the numbers In 2011, callers to 24/7 NurseLine were mostly women (62% overall). Studies show that women are more likely to be seekers of health information in a family. The highest users were 23 to 55 years old (52%), followed by 0 to 17 years old (23%). The top health topic was pediatrics (24%). Users also asked about digestion (12%), bone/muscle/joint (12%), dermatology (6%), neurology (6%) and cardiovascular (5%) issues. The other 34% fell under one of 15 other topics. The next time you see patients who are BCBSGa members, please tell them about the 24/7 NurseLine program. It’s a helpful resource any time they have a health issue. Future Moms promotes healthy pregnancies and healthy babies Our Future Moms program helps women have a healthy pregnancy, a safe delivery and a healthy baby. The program serves you and your patients through education, risk assessments a nd early interventions. Future Moms supports your plan of care for your patients and works with you to: Help them understand the importance of prenatal care and regular clinical visits. Encourage them to make better decisions about their health Spot risks and problems to ensure they get the help they need. Lower the risk of birth defects and low birth weight. How Future Moms supports you Maternity care providers benefit when we offer these services to their patients: Self-help resources so your patients can be well-informed about their health and care. This takes some of the burden off you. 24/7 phone access to a nurse. We do encourage members to talk to you about any concerns. But we take care of basic questions on health, benefits and care management so you don’t have to. Multilingual resources that help members who don’t speak English talk more easily with nurses. Resources include a Network Services Language Line, educational materials in Spanish and TTY capabilities for the speech- and hearing-impaired. Again, this takes some of the burden off you when language is a barrier. Help in authorizing and arranging for home health or other outpatient services. This frees up your time and ensures your patients get the extra care they need. Updates for you on the health status of high-risk members and proposed interventions. November 2013 16 of 39 How Future Moms helps members Future Moms guides women toward a healthy lifestyle from the start of the pregnancy until after the baby is born. Participants get: Help tracking the progress of the pregnancy from the first trimester through delivery. Each participant is placed in a high- or low-risk group based on health status and care needs. Customized support from nurses experienced in obstetrics. Participants work with a nurse care manager with at least three years of OB experience. 24/7 toll-free access to nurses. Participants can call with questions or concerns any time. Education on proper self-care, diet, signs of pre-term labor and other problems linked to pregnancy. Help on health issues that may affect their pregnancy. We offer support for conditions such as diabetes and high blood pressure. We also help participants with high-risk issues such as smoking, drug use, high stress levels and domestic matters. Help finding health care services and getting referrals. Follow-up support after the baby is born. The nurse: – Checks for postpartum depression. – Sends educational materials. – Refers members to behavioral health and EAP programs, social workers and lactation experts as needed. If you’d like to refer patients who are BCBSGa members into the program, please call 800-828-5891. You can also find a Future Moms referral form by going to the Health & Wellness page of our provider website, bcbsga.com. MyHealth Advantage works with you to help your patients MyHealth Advantage uses advanced technology to help our members follow your plan of care. The program helps improve patient health and coordination of care. We work with doctors to find and address medical gaps and health risks. How it works MyHealth Advantage scans medical, pharmacy and lab claims. We compare the information with current medical guidelines and best clinical practices. If we find health risks, members get a confidential message called MyHealth Note by mail. We suggest specific actions that when endorsed by their doctors can help the members improve their health. All MyHealth Notes tell members to speak with their doctors before making any changes in their medical care. MyHealth Note also includes a list of recent medical, lab and pharmacy claims that a member can share with you. These may be helpful for you in providing care, especially if members have other doctors. We keep you in the loop If we find a clinical issue, we let you know by mail, too. For example, if the member is overdue for an annual test or if there’s a drug therapy issue, we tell you. If you get a notice, you may add medical information about the member or about the recommendation. You may fax a form back or call a MyHealth Advantage representative. Messages are also posted on two websites, Availity, and MMH+. You can get real-time information when treating patients in the ER or in the office. MMH+ is a secure and free Internet site where you can check patient history. Availity is a payment website that shows benefit eligibility, which you can check before an office visit or procedure. Our goal is to offer you updated information to help you take care of your patients. Program benefits MyHealth Advantage alerts members if they can save money by switching to a generic drug. November 2013 17 of 39 The results of the program can include better health through improved compliance with medical standards. A recent study showed 46% of participants who received a MyHealth Note were brought back into clinical compliance. 1 The personal health guidance in MyHealth Note can help members understand their current health status. With MyHealth Note, members can make better decisions for their health and wellness. Currently, MyHealth Advantage is available to all our members except those with Medicaid coverage. 1 Based on an internal review of current participants; members acted within 12 months of getting the first MyHealth Note. E-business ProviderAccess is going away Nov. 8 th The transition of BCBSGa member eligibility and benefit information and claim status inquiry from ProviderAccess to the Availity Web Portal is almost here. The new address to access this valuable information is availity.com. You will not be able to access this information via ProviderAccess for Providers after November 8, 2013. We need you fully transitioned to the Availity Web Portal prior to shutdown.* Availity’s Web Portal currently offers BCBSGa providers access to the following functionality at no cost: Member eligibility and benefits inquiry – includes out-of-state BlueCard® members Claim status inquiry – includes out-of-state BlueCard members Claim submission – submit a single, electronic claim Secure messaging* – submit a question on a claim via a secured email to the appropriate provider inquiry area. Patient care summary (formerly known as CareProfile ® ) – real-time, consolidated view of a member’s medical history across multiple providers. Patient reminders (formerly clinical messaging) – clinical alerts on patients’ care gaps and medication compliance indicators. AIM Specialty Health SM (AIM) – link to precertification requests and inquiries through AIM . Member Certificate Booklet – view a local plan member’s certificate of coverage, when available. Online Remits* – link to online remits under Claims Management/Remittance Review. New! Interactive Care Reviewer – secure, online provider precertification tool. *The user must be registered with ProviderAccess for these roles. Registered but forgot your password? On theAvaility portal login page, click Help! I can’t login! Note: You must know the answers to the security questions you provided during initial login. Registered but locked out? Your PAA can unlock your access. If you don’t know who your PAA is contact 1.800.AVAILITY (282.4548) toll free (Monday – Friday 8:00a.m. – 7:00p.m.ET. Not sure if your organization is registered? Call Availity Client Services for registration status of your Tax ID. November 2013 18 of 39 You can make it even easier for your users to navigate between the Availity Web Portal and ProviderAccess. Here’s how! By entering each user’s ProviderAccess user ID in to BCBSGa Services Registration on Availity’s Web Portal and checking the box next to the access called BlueCross BlueShield Provider Portal, each user will be able to go to My Payer Portal/Blue Cross Blue Shield of Georgia Provider Portal on Availity and navigate to their ProviderAccess account without entering another log in and password. Free Training Once you log into the secure portal, you'll have access to many resources to help jumpstart your learning, including free liv e training, on-demand training, frequently asked questions, and comprehensive help topics. To view the current training resources, click Free Training at the top of any page in the Availity portal or click here to find a current schedule of FREE Availity workshops and webinars. Still need help? Contact Availity Client Services Call: 1-800-AVAILITY (282-4548) toll free Monday–Friday; 8:00a.m.–7:00p.m.ET E-mail: support@availity.com Don’t Delay, Get Registered for Availity Today! *Note: BCBSGa trading partners (providers, clearinghouses) exchanging electronic transactions via our Enterprise EDI Gateway are unaffected by our Provider Access functionalities moving exclusively to Availity on November 8, 2013. EDI trading partners will continue to use their existing EDI transmission channels to submit X12 transactions. Availity, an independent company, provides claims management services for Blue Cross and Blue Shield of Georgia. Interactive Care Reviewer (ICR) now accepts inpatient requests Our ICR tool continues to evolve, improving the precertification process. In the latest upgrade, new features now offer you the ability to submit both inpatient and outpatient precertfications online, plus ordering and servicing providers can submit an inquiry to find information on any precertification previously submitted via ICR. These are the most recent enhancements to our online precertification tool but not the last, so please stay tuned … In the meantime, if you have not already done so, we invite you to attend one of our upcoming informational webinars. To learn more about how you can streamline the precertification process by taking advantage of our ICR’s many features, register today by clicking here. As a reminder, you can access our ICR tool free of charge via the Availity Web Portal. If your organization has not yet registered for access, go to availity.com and click on Register Now. If your organization already has access to the Availity’Web Portal, your Primary Access Administrator can grant you acce ss to Authorizations and you can start using our tool right away. For questions regarding our ICR, please contact your local Network Management consultant. For questions on accessing our tool, call Availity Client Services at 800-AVAILITY (800-282-4548) or email questions to support@availity.com. Availity Client Services is available Monday-Friday, 8 a.m. to 7 p.m. ET (excluding holidays) to answer your registration questions. *Note: ICR is not currently available for Medicare Advantage, Medicaid, FEP, BlueCard®, and some National Account members; requests involving Behavioral Health or transplant services; or services administered by AIM Specialty Health SM. For these requests, follow the same precertification process that you use today. November 2013 19 of 39 IBM, the IBM logo, ibm.com, and Watson are trademarks of International Business Machines Corp., registered in many jurisdictions worldwide. Other product and service names might be trademarks of IBM or other companies. A current list of IBM trademarks is available on the Web at “Copyright and trademark information” at www.ibm.com/legal/copytrade.shtml State Health Benefit Plan Important information about the State Health Benefit Plan State Health Benefit Plan information is posted as it becomes available on the State Health Benefit Plan page on our provider website, bcbsga.com. State Health Network – SHBP The State Health Network – SHBP includes OpenAccess POS for members who are residents in the state of Georgia and BlueCard® PPO for members who are residing outside the state of Georgia. This network is used for all three HRA benefit options SHBP offers to its members. For all plans, covered in-network preventive care services will be paid at 100% for members. The three HRA metal level benefit options include the following: Gold: 85% is paid by Plan for approved in-network services; 60% is paid by Plan for approved out-of-network services. Silver: 80% is paid by Plan for approved in-network services; 60% is paid by Plan for approved out-of-network services. Bronze: 75% is paid by Plan for approved in-network services; 60% is paid by Plan for approved out-of-network services. Communications BCBSHP will be adding an SHBP specific section to our bi-monthly provider newsletter, Network Update, as well as adding a SHBP specific page to our provider website, bcbsga.com. We will also email late breaking important information via Network eUpdate (formerly Rapid Update). If you have not yet registered to receive Network eUpdate, please do so by visiting the Communications page of our provider website, bcbsga.com, or contact your Provider Representative for help. Webinars will be held in November. Precertification SHBP requires precertification for some services that are not required for non-SHBP members. This revised precertification list will be posted to the Precertification page and the SHBP page on our provider website, bcbsga.com. Providers must obtain precertification for the services listed in order to receive reimbursement. Future notifications of changes to the posted precertification list will be done through Network Update and posted to the Precertification page and the SHBP page of our provider website, bcbsga.com. AIM Specialty Health sm (AIM) Programs AIM programs include management of high-tech imaging, echocardiography, specialty pharmacy, radiation therapy and sleep studies and sleep therapy/treatment. All of these services require precertification. In addition, for providers of high -tech imaging services, sleep testing and sleep therapy/treatment, AIM requires the completion of an OptiNet SM online site assessment. The following AIM programs apply to SHBP: Diagnostic Imaging Program Imaging Cost and Quality Program Outpatient Radiation Therapy Program Sleep Management Program More information on the AIM programs can be accessed on the Answers@BCBSGa page, the Precertification page on our bcbsga.com provider website, by visiting AIM’s website at aimspecialtyhealth.com, or calling 800-252-2021. November 2013 20 of 39 Specialty Pharmacy SHBP has contracted directly with Express Scripts, Inc (ESI) as its ph armacy vendor. The list of pharmaceuticals that will be included in the pharmacy benefit program, managed by ESI, will be posted to the SHBP page of our provider website, bcbsga.com. Contact information can also be found on the SHBP page. Claim Processing Certain benefits, as defined by SHBP, will require specific standard industry codes in order for the service to be considered a covered benefit. Claim submission requirements will be posted to the bcbsga.com provider website. SHBP Contact Information SHBP has a designated Provider Customer Service department and address. SHBP Provider Customer Service can be reached the following ways: Any SHBP related claims or correspondence should be mailed to: Phone: 855-641-4862 Blue Cross and Blue Shield of Georgia P.O. Box 105370 Atlanta, GA 30348-5370 For questions regarding the information in this letter, please contact SHBP Provider Cu stomer Service at 855-641-4862, Provider Relations at 888-706-3475, or your local Provider Representative. Senior business and Medicare Advantage Please ensure referred physicians are in the Medicare Advantage Network When referring a BCBSGa Medicare Advantage member to a specialist, please ensure that the physician participates in the member’s health plan. Depending on the member’s benefit plan, services may not be covered or a significant member cost share may exist when seeking services from a non-participating provider. You can use Find a Doctor at bcbsga.com to make sure the referral is to an in-network provider. Physicians to receive home test kits results for Medicare Advantage members Medicare Advantage members who appeared to be missing key tests or screenings for colorectal cancer, blood sugar, and cholesterol screenings began receiving a home test kit from us in August and will receive these home test kits as needed through the end of the year. Providers will receive a letter from us detailing the member’s test results. Please review the t est results and discuss the member as needed. Record exact adult BMI number, not range Healthcare Effectiveness Data and Information Set (HEDIS ®) is updating the medical records specifications for Adult Body Mass Index (BMI). In the past, it was acceptable to record a range for BMI, such as >30. In 2014, HEDIS specifies that the exact BMI number should be recorded in the medical record, such as 32. Greater precision in charting the member’s BMI will help the provider help the member achieve or remain at a healthy weight. November 2013 21 of 39 Update: Avoidable Medicare Advantage readmissions may lead to administrative claim denials To achieve the best possible quality of care outcomes f or our Medicare Advantage members and in support of the clinical quality issue that is being driven by CMS’ Readmission Quality Improvement Program (QIP), we have an obligation to review readmissions for clinical relatedness. In accordance with the Diagno sis Related Groups (DRG) payment methodology, WellPoint will be following a uniform 30-Day Readmission Review Program for our Medicare Advantage program that is consistent with Centers for Medicare & Medicaid Services (CMS) quality improvement guidance. 1 Payment for a Medicare Advantage member’s DRG readmission to the same acute facility within 30 days of the first admission may be administratively denied if, the readmission is determined to be related to the previous admission. This Program will apply to facilities’ claims only. We will not administratively deny professional or ancillary claims based upon the 30-Day Readmission Review Program. The administrative denial of these claims will apply to participating and non participating facilities. 2 As a reminder, all claims denied administratively under this Readmission Review Program are denied as provider liability. Our member is not liable for these denied claims. Providers are not permitted to balance bill the memb er for the denied claim. Additional information can be found in the BCBSGa Medicare Advantage Guidebook. Please share this information with clinical staff and others involved with admissions. 1. Chapter 4, Section 4240 (Readmission Review) of the Medicare Quality Improvement Organization Manual: Readmission review involves admissions to an acute, general, short-term hospital occurring less than 31 calendar days from the date of discharge from the same or another acute, general, short-term hospital (See §1154(a)(13) and 42 CFR 476.71(a)(8)(ii)). Medicare QIO Manual, Chapter 4, Section 4240: Perform case review on both stays. Analyze the cases specifically to determine whether the patient was prematurely discharged from the first confinement, thus causing readmission. Perform an analysis of the stay at the first hospital to determine the cause(s) and extent of any problem(s) (e.g., incomplete or substandard treatment). Consider the information available to the attending physician who discharged the patient from the first confinement. Do not base a determination of a premature discharge on information that the physician or provider could not have known or events that could not have been anticipated at the time of discharge. 2. Subject to explicit language that sets forth a different payment methodology Medicare reports on osteoporosis management after bone fracture Once a woman has had a fracture, she has a four times greater risk of another fracture, reports the National Institute of Arthritis and Musculoskeletal and Skin Diseases. To monitor osteoporosis management, the National Committee for Quality Assurance reports to Medicare which of your female patients 67 years old or older has had a fracture and has had either bone mineral density testing or medication to treat or prevent osteoporosis within six months of the fracture. Screening and treatment can significantly improve health outcomes by preventing f ractures. Osteoporosis therapy may reduce the risk of fracture by nearly 50 percent, according to the Journal of Rheumatology. Medicare-covered bone density scans are covered with no cost share to member. With some plans, there may be a cost share if the member receives these services out of network. All qualified people with Medicare who are at risk for osteoporosis and meet one of the following five criteria are eligible: A woman whose doctor determines she's estrogen deficient and at risk for osteo porosis, based on her medical history and other findings November 2013 22 of 39 A A A A person person person person whose X-rays show possible osteoporosis, osteopenia, or vertebral fractures taking prednisone or steroid-type drugs or is planning to begin this treatment who has been diagnosed with primary hyperparathyroidism who is being monitored to see if their osteoporosis drug therapy is working Medicare Advantage Part D formulary changing for 2014 Please help members with new choices Each year we evaluate our benefits and formulary and may make changes to update them. Formulary changes in the upcoming year include: tier changes, drug removals, and new Prior Authorization and Quantity Limit requirements. Members will have formulary changes and will need your help to ensure they get their needed treatments at the most affordable cost. For your insulin dependent diabetic patients, it is important to note that many of BCBSGa’s plans will be removing the Novolin line of products from formulary. BCBSGa is communicating thi s change directly to impacted members by letter and phone encouraging them to contact their provider regarding this change in coverage. Please encourage members to review the 2014 formulary information within their Annual Notice of Change (ANOC) mailing, or to view the information online. Ask them if the coverage for any of their prescriptions has been changed, and consider alternative medications in a lower cost-sharing tier that may meets their need. 2014 Medicare Advantage coverage changes for diabetic supplies Beginning January 1, 2014, our Individual Medicare Advantage plans will only cover LifeScan, Inc., OneTouch ® or Roche Diagnostics, ACCU-CHEK ® diabetic glucometers and blood test strips at a $0 copay when the members purchases their supplies from an in-network supplier. This benefit change is meant to help control out-of-pocket expenses while not compromising on quality. Covered glucometers and blood test strips in 2014: LifeScan, Inc., OneTouch ® Roche Diagnostics, ACCU-CHEK ® A limit of 100 blood test strips per month Next steps If our member is currently using OneTouch or ACCU-CHEK blood test strips or glucometer products, you don’t need to do anything! If our member is not using OneTouch or ACCU-CHEK blood test strips or glucometer products, then our member will need to get new prescriptions for the supplies by January 1st for these claims to be covered by us. You should discuss these coverage changes and possible new prescriptions with our member/your patient. If it is medically necessary for them to continue using a different brand of blood test strips or glucometer and/or more than 100 blood test strips per month, you will need to communicate this to us by requesting an exception. If your patient purchases their supplies through the pharmacy or the ESI mail-order service exceptions may be requested after December 1, 2013 by calling 800-338-6180. If your patient purchases their supplies through a Durable Medical Equipment supplier you will need to call the health plan. – 866-797-9884 (call for Diabetic brand glucometer/Quantity limit on test strips) – 800-959-1537 (fax for Diabetic brand glucometer/Quantity limit on test strips) November 2013 23 of 39 Please contact Provider Service if you have any questions about these coverage changes or your patient’s benefits. Plans that are included in this coverage change include: CONTRACT # Plan Benefit Package (PBP) PLAN NAME H5422 002 BlueValue Secure (HMO) H5422 006 BlueValue Basic (HMO) H9947 001 Medicare Preferred Core (PPO) PLAN TYPE GA-HMO GA-HMO GA-LPPO BCBSGa is a PPO plan with a Medicare contract. Enrollment in BCBSGa depends on contract renewal. If a member or a provider is in doubt as to whether or not a member is in an Individual Medicare Advantage Prescription Drug (MAPD) plan or an Employer or Union Sponsored plan, please have them check the front of the ID card which will show the contract and PBP number (example: H1234-001). If the PBP (the last three digits of the contract-PBP number) is in the 800 series, that member is in an Employer or Union Sponsore d plan and these changes do not apply to their plans. The benefit information provided is a brief summary, not a complete description of benefits. For more information contact the plan. Limitations, copayments, and restrictions may apply. Benefits, formul ary, pharmacy network, provider network, premium and/or co-payments/co-insurance may change on January 1st of each year. For more information about the exception process or the appeals policy, please see the plan’s 2014 Evidence of Coverage. American Diabetes Association offers medication guidelines for patients with diabetes/hypertension The Centers for Medicare & Medicaid Services (CMS) tracks several performance and quality measures in place for Medicare Part D members. To ensure that we are in alignme nt with CMS, one of the measures we’ll be focusing on for the remainder of this year is medication treatment for patients with diabetes and hypertension. The American Diabetes Association guidelines recommend that ACE inhibitors or ARB medications be give n to patients with diabetes and hypertension to help reduce the risk of cardiovascular events and the progression of nephropathy indicated by levels of microalbuminuria/albuminuria. If your patients have hypertension, we ask that you please consider whethe r an ACE inhibitor or ARB medicine may be an appropriate treatment at this time. Based on the American Diabetes Association guidelines, we offer you this information with the hope that you may find it useful in your efforts to help ensure high-quality care for your patients. Speaking the Language of ICD-10 - Part 2 In the last publication, as part of our implementation ICD-10 efforts, we discussed the critical role complete and accurate medical record documentation and diagnosis coding plays in managing o ur Medicare Advantage membership. Remember, your coding and record documentation efforts have a direct impact on accurate risk adjusted payment, as well as the services and benefits BCBSGa is able to provide to our membership. ICD-10 Preparation You and your coding staff do not have to learn the entire code set. One way to help your practice prepare for the upcoming ICD-10 changes is to review your documentation for the most commonly used ICD -9 codes in your practice. You can then begin working with your coding staff to select the appropriate corresponding ICD-10 codes. Identifying the challenges now November 2013 24 of 39 will help reinforce the documentation requirements in order for your coders to assign the most appropriate diagnoses code for ICD-10. This may also provide you with a tool for assessing your staff and training needs. As a guideline, coding professionals recommend that any required coding training take place approximately six months prior to the ICD -10 compliance deadline. ICD-10 Impact on Clinical Documentation While ICD-10 will not change the way you provide care to your patient, the amount of details documented in the medical record for your coding staff will need to be expanded. Specificity will be a key component to ICD -10 documentation. It will be even more important to ensure you are documenting to the highest degree of specificity. For example, in ICD-10, the selection of laterality is expanded. Clinical documentation for the diagnosis should include information as to which side of the body is affected (i.e., right, left, or bilateral). BCBSGa realizes that concepts that are new to ICD-10 may not be new to you. However, to assist you with your preparation, you will find an example of what to include in your documentation for a fracture diagnosis to ensure your coders can accurately assign ICD-10 codes: To code for the diagnosis of a fracture in ICD-10, the following details will need to be provided: Site Laterality Type Location For traumatic fracture, the ICD-10 code set has the following 7 th character selection: A Initial encounter for closed fracture B Initial encounter for open fracture D Subsequent encounter for fracture with routine healing G Subsequent encounter for fracture with delayed healing K Subsequent encounter for fracture with nonunion P Subsequent encounter for fracture with malunion S Sequela In addition to providing details for the diagnosis of the fracture, it is important to also document how the injury occurred and where. For accurate code assignment, provide the following information for injuries: External cause – Provide the cause of the injury; when meeting with patients, ask and document “how” the injury happened. Place of occurrence – Document where the patient was when the injury occurred; for example, include if the patient was at home, at work, in the car, etc. Activity code – Describe what the patient was doing at the time of the injury; for example, was he or she playing a sport or using a tool? External cause status – Indicate if the injury was related to military, work, or other. This example was provided as acute injury codes are included in the 2013 HCC Model for risk adjustment. As you can see from this example, fracture is one of the diagnoses that have expanded the level of specificity required to code the fracture which includes the activity codes involved. In our next article in this 3-part series our focus will be on specific diseases, providing you with suggestions and recommendations to help you prepare for the transition to ICD-10. We at BCBSGa have been busy transforming our business as well in preparation for this upcoming transition, not only for working toward the ICD -10 deadline requirement, but to also November 2013 25 of 39 add value and innovation. You will also find some valuable information on the CMS website and American Medical Association website. Pharmacy Pharmacy information available on bcbsga.com Visit anthem.com/pharmacyinformation for more information on pharmacy copayment/ coinsurance requirements and their applicable drug classes, Drug Lists and prior authorization criteria, procedures for generic substitution, t herapeutic interchange, step therapy or other management methods subject to prescribing decisions, and any other requirements, restrictions, or limitations that apply to using certain drugs. Coverage for compound drugs Due to the recent enhancement of the HIPPA standard for electronic submission of prescription drug claims we now have the ability to better administer our drug benefits as they pertain to compounded drugs. During a recent review, we learned that claims for certain compounded drugs have been submitted and paid as a prescription drug benefit. For a compound drug to be covered it must contain at least one ingredient/drug that requires a prescription to obtain. Additionally, that ingredient/drug must also be approved by the Food and Drug Administ ration (FDA). Claims for certain compound drugs currently being paid will no longer be paid effective November 1, 2013. These include: Compounded bulk powders (not FDA approved) Pharmaceutical Adjuvants (compounding vehicles, not FDA approved) Letters have been mailed to impacted members. Please note: We will still cover compound drugs whose primary ingredient is FDA -approved and not otherwise excluded, as defined under the plan. Policy updates Always Bundled Policy: Certain bundled services ineligible for separate reimbursement There are services and supplies that are always considered part of providing another service and therefore are not eligible for separate reimbursement when reported by a professional provider. These bundled services may be performed or provided either on the same or different date of service as the primary service. The services listed below are being added to BCBSGa’s Bundled Services and Supplies policy effective January 1, 2014: Codes A4216 Sterile water, saline and/or dextrose, diluent/flush, 10 ml A4218 Sterile saline or water, metered dose dispenser, 10 ml A4264 Permanent implantable contraceptive intratubal occlusion device(s) and delivery system November 2013 26 of 39 Please refer to the Always Bundled reimbursement policy to inform of services and supplies not eligible for separate reimbursement when billed with another specific procedure or service. The following identifies some of the procedures that are described in Section 2 of the Always Bundled reimbursement policy. The exclusion of a specific code does not indicate eligibility for reimbursement under all circumstances. These code relationships are proved as an informational tool only to help identify some of the procedures described within the policy. A4264 (or a reported unlisted code such as L8699) with the occlusion of the fallopian tubes by hysteroscopy code 58565 Following Current Procedural Terminology (CPT®) reporting guidelines, 76942 (Ultrasonic guidance for needle placement (eg, biopsy, aspiration, injection, localization device), imaging supervision and interpretation) is not eligible for separate reimbursement when reported with any of the procedure codes CPT identifies 76942 should not be separately with. The Always Bundled updated reimbursement policy will be available online to view by December 1, 2013. View BCBSGa Professional Reimbursement policies at bcbsga.com If you are not a registered secure provider portal user on the bcbsga.com provider website you will need to register before you can view the Professional Reimbursement policies. In the left corner of the Provider Home Page is an option to register. Complete the registration form and your ID and Password will be mailed to you within two weeks. If you are a registered bcbsga.com secure provider portal user, go to left side of the screen and select Login, enter login and password, select Policy and Procedures tab, select the link labeled Reimbursement Policies. Medical Policy and Clinical Guideline updates The Medical Policy and Technology Assessment Committee adopted the following new and/or revised Medical Policies and Clinical Guidelines. Some may have expanded rationales, medical necessity indications or criteria and some may involve changes to policy position statements that might result in services that previously were covered being found to be either not medically necessary or investigational/not medically necessary. Clinical Guidelines adopted by Blue Cross Blue Shield and all the Medical Policies are available at bcbsga.com. Please note that our medical policies now include NOC (Not Otherwise Classified) codes to expedite the process of determining services that may require medical review. If you do not have access to the Internet, you may request a hard copy of a specific Medical or Behavioral Health Policy or Clinical UM Guideline by calling Provider Services at (800) 241-7475 Monday–Friday from 8:00 a.m. to 7:00 p.m. or send written requests (specifying the medical policy or guideline of interest, your name and address to where the information should be sent) to: BCBSGa Attention: Prior Approval, MC: GAG009-0002 3350 Peachtree Road NE Atlanta, GA 30326 AIM Specialty Health SM (AIM) To submit your request for any of the services below, contact AIM online via A IM’s ProviderPortal SM at aimspecialtyhealth.com/goweb. From the drop down menu, select BCBSGa. You may also call AIM toll free at 866 -714-1103, Monday–Friday, 8:00 a.m.–6:00 p.m. Diagnostic Imaging Management Diagnostic imaging management services are provided AIM, a separate company, for certain health plan members. Diagnostic imaging services may be reviewed against AIM’s Diagnostic Imaging Utilization Management Clinical Guidelines. July 2013 27 of 35 AIM’s clinical guidelines are available on their website. If you have any questions about which guidelines are applicable, please call the customer service number on the back of the member’s ID card. November 2013 27 of 39 Radiation Therapy Services Effective November 1, 2012, BCBSGa transitioned the review of outpatient radiation therapy services to AIM. AIM is a nationally recognized leader in specialty benefits management. Providers must contact AIM for prior authorizat ion for the following non-emergency outpatient services: Intensity Modulated Radiation Therapy (IMRT), Proton Beam Radiation Therapy, Stereotactic Radiosurgery (SRS)/Stereotactic Body Radiotherapy (SBRT) and Brachytherapy. Radiation therapy performed as part of an inpatient admission will continue to be reviewed through the BCBSGa’s inpatient precertification process. Members who were already undergoing treatment on November 1, 2012 are not impacted. Prior authorization is required through AIM for all BCBSGa members, with the exception of members with Medicare supplemental policies, Medicare Advantage plans, BCBSGa as secondary coverage and the Federal Employee Program. Outpatient Sleep Testing and Therapy Services A specialty benefit management program for outpatient sleep testing and therapy services for obstructive sleep apnea became effective on November 1, 2012. This program is administered by AIM and includes the following: Home sleep test (HST) In-lab sleep study (PSG) Titration study Initial treatment order (APAP, CPAP, BPAP, oral devices, appliances and related supplies) Ongoing treatment order (APAP, CPAP, BPAP, oral devices, appliances, and related supplies) BCBSGa uses sleep diagnostic and treatment guidelines developed by AIM. AIM’s Obstructive Sleep Apnea Diagnostic & Treatment Management Guidelines are available at aimspecialtyhealth.com/gowebsleep. Effective November 1, 2012, the precertification requirement applies to BCBSGa members who participate in BCBSGa local and individual health plans. Effective January 1, 2013, it also applies to members covered by Medicare Advantage. The requirement does not apply to those in the Federal Employee Program (FEP) and those for whom BCBSG a is secondary coverage including those whose primary insurance carrier is Medicare. By clicking on the links above, you will be linked to sites created and/or maintained by another, separate entity (“External Site”). Upon linking you are subject to the terms of use, privacy, copyright and security policies of the External Sites. We provide these links solely for your information and convenience. We encourage you to review the privacy practices of the External Sites. The information contained on the External Sites should not be interpreted as medical advice or treatment provided by us. Effective Policy or Guideline Number Date Title and Summary NEW MEDICAL POLICIES AND CLINICAL UM GUIDELINES 2/1/2014 DME.00037 Cooling Devices and Combined Cooling/Heating Devices This policy addresses both passive and active cooling devices, as well as devices that combine compression or heat therapy for the relief of pain and swelling due to trauma, surgery and other conditions. This policy does not address the use of whole body or head cooling devices for adult or pediatric individuals with acute neurologic injury or after sudden cardiac death. 2/1/2014 DRUG.00055 Denosumab (Prolia®, Xgeva™) This policy addresses the use of denosumab which is a subcutaneous, fully human monoclonal antibody that is specifically designed to target the human receptor activator of nuclear factor kappa -B ligand (RANKL) for the treatment of individuals with osteoporosis, treatment induced bone loss, bone metastases, giant cell tumor of the bone, and for all other indications, including multiple myeloma and rheumatoid arthritis which are currently being studied. November 2013 28 of 39 2/1/2014 DRUG.00057 Canakinumab (Ilaris®) This policy addresses the indications for use of canakinumab which is a humanized monoclonal antibody, interleukin-1 beta (IL-1 ß) inhibitor drug that works by binding human IL-1ß and neutralizes its activity by blocking its interaction with IL-1 receptors. 2/1/2014 DRUG.00058 Pharmacotherapy for Hereditary Angioedema This policy addresses four drugs that have been specifically developed for the treatment or prevention of hereditary angioedema (HAE) attacks. Berinert® and Cinryze ® (both C1 -esterase inhibitor, human) supplement deficient or defective C1- esterase-inhibitor (C1-INH). Kalbitor® (ecallantide) and Firazyr® (icatibant) act by inhibiting kallikrein or blocking bradykinin receptors which are the primary mediators for HAE. 2/1/2014 LAB.00030 Measurement of Serum Concentrations of Infliximab (IFX) or Antibodies -to-Infliximab (ATI) This policy addresses the measurement of serum concentrations of infliximab (IFX) and antibodies -toinfliximab (ATI) in individuals with various conditions. Such te sting has been proposed as a way to detect individuals with poor or lack of response to infliximab treatment with the goal of altering treatment to optimize outcomes. 10/8/2013 CG-BEH-03 Psychiatric Disorder Treatment This guideline addresses psychiatric disorder treatment for: Acute Inpatient; Residential Treatment Center (RTC); Partial Hospitalization Program (PHP); Intensive Structured outpatient Program (IOP); Inpatient/Outpatient Electroconvulsive Therapy (ECT). 10/8/2013 CG-BEH-04 Substance Abuse Treatment This guideline addresses substance abuse treatment for: Inpatient Acute Detoxification; Inpatient Acute Rehabilitation; Residential Treatment Detoxification; Residential Treatment Center (RTC); Partial Hospitalization Program (PHP); Intensive Structured Outpatient Program (IOP); Outpatient Treatment; Outpatient Detoxification Outpatient Treatment With Extended On -Site Monitoring; Outpatient Detoxification Without Extended On -site Monitoring (Office Based); Outpatient (Office Based) Medication Assisted Treatment (MAT) of Opioid Dependence. 10/8/2013 CG-BEH-05 Eating Disorder Treatment This document addresses eating disorder treatment for: Acute Inpatient; Residential Treatment Center (RTC); Residential Treatment Center (RTC) without 24 Hour Nursing; Partial Hospitalization Program (PHP); November 2013 29 of 39 Intensive Structured Outpatient Program (IOP); Outpatient. 10/8/2013 CG-BEH-06 Psychiatric Outpatient Treatment This guideline addresses psychiatric outpatient treatment (including treatmen t provided by a clinician licensed at the independent practice level) and medication management. 10/8/2013 CG-BEH-07 Psychological Testing This guideline addresses psychological testing. 10/8/2013 CG-BEH-08 Employee Assistance Program Outpatient Treatment This guideline addresses Employee Assistance Program (EAP) Outpatient Treatment Criteria and these criteria apply only to California DMHC Regulated Business. 2/1/2014 CG-DME-35 Breastfeeding Pumps This guideline addresses the non-standard, electric, heavy-duty, hospital-grade breast pump for initiating and maintaining expression of human breast milk in specified situations. 2/1/2014 CG-SURG-34 Diagnostic Infertility Surgery This guideline addresses the use of hysteroscopy and laparoscopy for diagn ostic work-up of infertility. 2/1/2014 CG-SURG-35 Intracytoplasmic Sperm Injection (ICSI) This guideline addresses the use of intracytoplasmic sperm injection (ISCI) during an infertility treatment cycle, allowing couples with male factor infertility to attain live birth rates, similar to those achieved with in vitro fertilization (IVF) using conventional methods of fertilization. REVISIONS TO EXISTING MEDICAL POLICIES OR CLINICAL UM GUIDELINES 10/8/2013 ADMIN.00001 Medical Policy Formation This policy describes the medical policy formation process. 10/8/2013 ADMIN.00002 Preventive Health Guidelines This policy provides links to several national organizations' evidence -based guidelines for preventive services. 10/8/2013 ANC.00009 Cosmetic and Reconstructive Services of the Trunk and Groin This policy addresses a variety of surgical procedures of the trunk or groin that may be considered medically necessary, cosmetic or reconstructive in nature. November 2013 30 of 39 10/8/2013 BEH.00004 Behavioral Health Treatments for Autism Spectrum Disorders and Rett Syndrome This policy addresses pharmacotherapeutic and behavioral health -related treatments and therapies used to treat Autism Spectrum Disorders (ASDs) and Rett syndrome. Previously titled: Behavioral Health Treatments for Pervasive Developmental Disorders 10/8/2013 DME.00027 Ultrasound Bone Growth Stimulation This policy addresses the use of low-intensity pulsed ultrasound devices as a treatment to promote healing of some fresh fractures and to accelerate healing for nonunion of other fracture sites. 8/12/2013 DRUG.00002 Tumor Necrosis Factor Antagonists This policy addresses the indications for a class of biologic disease -modifying antirheumatic drugs (DMARDs) known as tumor necrosis factor (TNF) ant agonists (inhibitors), that target specific pathways of the immune system and either enhance or inhibit immune response. 8/12/2013 DRUG.00015 Prevention of Respiratory Syncytial Virus Infections This policy identifies populations at high risk for RSV a nd indications for RSV prophylaxis. 8/12/2013 DRUG.00038 Bevacizumab (Avastin®) for Non-Ophthalmologic Indications This policy addresses the indications and criteria for the use of bevacizumab in the treatment of oncologic conditions and other non-ophthalmologic indications. 8/12/2013 DRUG.00043 Tocilizumab (Actemra®) This policy addresses the use of tocilizumab for the treatment of adults with moderately to severely active rheumatoid arthritis (RA) and children two years of age and older with active pol yarticular juvenile idiopathic arthritis (PJIA) or active systemic juvenile idiopathic arthritis (SJIA). 10/8/2013 DRUG.00044 Belimumab (Benlysta®) This policy addresses the use of belimumab for the treatment of individuals age 18 or older with active, antibody-positive systemic lupus erythematosus (SLE), and for other indications. 2/1/2014 GENE.00001 Genetic Testing for Cancer Susceptibility This policy addresses genetic testing for individuals who are at higher than average risk for the development of cancer. 8/12/2013 MED.00080 Cryopreservation of Oocytes or Ovarian Tissue This policy addresses oocyte and ovarian tissue cryopreservation which are alternative techniques to embryo cryopreservation for women who would become infertile due to gonadotoxi c therapies such as, chemotherapy, radiation therapy or surgery. 10/8/2013 MED.00107 Medical and Other Non-Behavioral Health Related Treatments for Autism Spectrum Disorders and Rett Syndrome This policy addresses pharmacotherapeutic, medical, and clini cal rehabilitative treatments and therapies used to treat Autism Spectrum Disorders (ASDs) and Rett syndrome. Previously titled: Medical and Other Non-Behavioral Health Related Treatments for Pervasive Developmental Disorders November 2013 31 of 39 2/1/2014 RAD.00035 Coronary Artery Imaging: Contrast-Enhanced Coronary Computed Tomography Angiography (CCTA), Coronary Magnetic Resonance Angiography (MRA), and Cardiac Magnetic Resonance Imaging (MRI) This policy addresses contrast-enhanced computed tomography angiography (CTA) of the coronary arteries (coronary CTA or CCTA), magnetic resonance angiography (MRA) and magnetic resonance imaging (MRI) of the coronary arteries. 10/8/2013 SURG.00007 Vagus Nerve Stimulation This policy addresses the use of an implantable vagal nerve s timulation (VNS) device and the electronic analysis of the implanted neurostimulator pulse generator system for the treatment of medically and surgically refractory seizures associated with intractable epilepsy and as a treatment of other conditions. 10/8/2013 SURG.00011 Allogeneic, Xenographic, Synthetic and Composite Products for Wound Healing and Soft Tissue Grafting This policy addresses the use of soft tissue (e.g., skin, ligament, cartilage, etc.) substitutes in wound healing and surgical procedures. 8/12/2013 SURG.00017 Stereotactic Radiosurgery (SRS) and Stereotactic Body Radiotherapy (SBRT) This policy addresses stereotactic radiosurgery (SRS) and stereotactic body radiotherapy (SBRT) which are non invasive treatments where high doses of focuse d radiation beams are precisely delivered to intracranial and extracranial targets, thus sparing adjacent tissue and structure from irradiation. 2/1/2014 SURG.00037 Treatment of Varicose Veins (Lower Extremities) This policy addresses various modalities for the treatment of valvular incompetence (i.e., reflux) of the greater or lesser saphenous veins and associated varicose tributaries as well as telangiectatic dermal veins. 10/8/2013 SURG.00049 Mandibular/Maxillary (Orthognathic) Surgery This policy addresses medically necessary, reconstructive and cosmetic procedures involving the mandible, maxilla or both, with the exception of orthognathic surgery for the treatment of temporomandibular disorders or obstructive sleep apnea. 8/12/2013 SURG.00055 Cervical Artificial Intervertebral Discs This policy addresses the use of U.S. Food & Drug Administration (FDA) approved cervical artificial intervertebral discs as a treatment for symptomatic cervical disc disease when conservative treatment options have been unsuccessful. 10/8/2013 SURG.00064 Cardiac Resynchronization Therapy (CRT) with or without an Implantable Cardioverter Defibrillator (CRT/ICD) for the Treatment of Heart Failure This policy addresses biventricular cardiac pacing to deliver cardiac r esynchronization therapy (CRT) to alleviate the symptoms of moderate to severe congestive heart failure (HF) associated with left ventricular dyssynchrony. It also addresses a hybrid device that combines CRT with an implantable cardioverter defibrillator (ICD). 10/8/2013 SURG.00071 Percutaneous and Endoscopic Spinal Surgery This policy addresses percutaneous and endoscopic spinal discectomy and disc decompression as well as image-guided minimally invasive lumbar decompression procedures. November 2013 32 of 39 10/8/2013 SURG.00077 Uterine Fibroid Ablation: Laparoscopic or Percutaneous Image Guided Techniques This policy addresses laparoscopic and percutaneous ablative techniques for the treatment of symptomatic uterine fibroids. Previously titled: Laparoscopic and Percutan eous MRI-Image Guided Techniques for Myolysis as a Treatment of Uterine Fibroids 10/8/2013 SURG.00117 Sacral Nerve Stimulation (SNS) and Percutaneous Tibial Nerve Stimulation (PTNS) for Urinary and Fecal Incontinence; Urinary Retention This policy addresses sacral nerve stimulation (SNS) and percutaneous tibial nerve stimulation (PTNS) in those with chronic, refractory urinary and fecal incontinence, as well as urinary retention. 8/12/2013 SURG.00122 Venous Angioplasty with or without Stent Placement This policy addresses the use of venous angioplasty as a treatment modality. 10/8/2013 CG-BEH-01 Assessment for Autism Spectrum Disorders and Rett Syndrome This guideline addresses various tools used in the screening and assessment of individuals with suspected Autism Spectrum Disorders (ASDs) and Rett syndrome. Previously titled: Assessment for Pervasive Developmental Disorders 10/8/2013 CG-DRUG-11 Infertility Drugs This guideline addresses the use of oral, injectable and topical infertility drugs, including protocols used to treat women with ovulation disorders, drugs used as part of an Assisted Reproductive Technology (ART) treatment (most commonly through in vitro fertilization [IVF]), intrauterine insemination, and as treatment of male infertility with gonadotropins. 10/8/2013 CG-DRUG-28 Alglucosidase alfa (Lumizyme®, Myozyme®) This guideline addresses alglucosidase alfa products (Lumizyme and Myozyme which are enzyme replacements used for specific indications as a treatment of Pompe disease. 8/12/2013 CG-MED-26 Neonatal Levels of Care This guideline addresses therapies and services provided in AAP defined level of care required for the normal healthy newborn to the critically ill newborn. 10/8/2013 CG-MED-44 Holter Monitors This guideline addresses the use of the standard external ambulatory Holter monitor. 2/1/2014 CG-MED-49 Auditory Brainstem Responses (ABRs) and Evoked Otoacoustic Emissions (OAEs) for Hearing Disorders This guideline addresses auditory brainstem response (ABR) and ev oked otoacoustic emission (OAE) testing which are noninvasive methods used to detect hearing disorders. Previously titled: Auditory Brainstem Responses (ABRs) and Evoked Otoacoustic Emissions (OAEs) for Screening and Diagnosis of Hearing Disorders 10/8/2013 CG-SURG-24 Functional Endoscopic Sinus Surgery (FESS) This guideline addresses the use of functional endoscopic sinus surgery (FESS), an endoscopic surgical November 2013 33 of 39 procedure used to treat various conditions of the nasal sinuses, including but not limited to chronic sinusitis. 10/8/2013 CG-SURG-27 Gender Reassignment Surgery This guideline addresses gender reassignment surgery which is one treatment option for extreme cases of gender dysphoria, a condition in which a person feels a strong and persistent ide ntification with the opposite gender accompanied with a severe sense of discomfort in their own gender. REVIEW OF EXISTING MEDICAL POLICIES OR CLINICAL UM GUIDELINES No change BEH.00002 to previous Transcranial Magnetic Stimulation for Depression and Other Neuropsychiatric Disorders effective date This policy addresses transcranial magnetic stimulation (TMS) as a treatment of behavioral health indications including depression and other neuropsychiatric disorders. 10/1/2013 10/8/2013 GENE.00021 Cytogenomic Microarray Analysis (CMA) for Developmental Delay, Autism Spectrum Disorder and Mental Retardation This policy addresses cytogenomic microarray analysis (CMA), also known as array comparative genomic hybridization (aCGH) and cytogenomic constitutional (genome-wide) microarray analysis, which is used as a diagnostic tool for individuals with unexplained developmental delay (DD), autism spectrum disease (ASD) and mental retardation (MR). Previously titled: Comparative Genomic Hybridization (CGH) Microarray Testing for Developmental Delay, Autism Spectrum Disorder and Mental Retardation 10/8/2013 LAB.00027 Antigen Leukocyte Cellular Antibody Test (ALCAT) for Chemical and Food Allergies This policy addresses ALCAT which measures whole bloo d leukocyte activity to identify allergens which cause an increase in the leukocyte activity. The ALCAT has been promoted as a diagnostic test for food allergy or intolerance (chemical sensitivities) and as a tool to establish elimination diets. No change LAB.00029 to previous AmniSure® ROM (Rupture of Membranes) Test effective date 12/2/2013 This policy addresses the AmniSure® test which detects PAMG -1 (placental alpha-1 microglobulin) protein marker of amniotic fluid in vaginal secretions and is inten ded for use by health care professionals as a point of-care service to aid in the detection of rupture of membranes (ROM) in pregnant women with signs, symptoms or complaints suggestive of ROM. 10/8/2013 MED.00064 Transcatheter Ablation of Arrhythmogeni c Foci in the Pulmonary Veins as a Treatment of Atrial Fibrillation (Radiofrequency and Cryoablation) This policy addresses transcatheter radiofrequency ablation and cryoablation of arrhythmogenic foci in the pulmonary veins for the treatment of atrial fibrillation. 12/2/2013 MED.00112 Autonomic Testing This document addresses the use of autonomic testing. Autonomic nervous system testing can be grouped into three categories; sudomotor, cardiovagal innervation, and vasomotor adrenergic innervation. The tests for sudomotor function can include QSART, TST, SSR, Silasticsweat imprint, and QDIRT. This document November 2013 34 of 39 does not address the use of tilt -table testing. 10/8/2013 RAD.00063 Magnetization-Prepared Rapid Acquisition Gradient Echo Magnetic Resonance Imagi ng (MPRAGE MRI) This document addresses magnetization-prepared rapid acquisition gradient echo (MPRAGE) MRI for all indications including the assessment of carotid artery plaque. 10/8/2013 SURG.00047 Transendoscopic Therapy for Gastroesophageal Reflux D isease and Dysphagia This policy addresses selected transendoscopic therapies for the treatment of gastroesophageal reflux disease (GERD) and dysphagia. This document does not address procedures which approach the esophagus through abdominal laparoscopic or open surgical approaches. 10/8/2013 SURG.00066 Percutaneous Neurolysis for Chronic Neck and Back Pain This policy addresses percutaneous techniques to treat spinal pain identified as being facet in origin. 10/8/2013 SURG.00089 Balloon Sinus Ostial Dilation This policy addresses the use of balloon sinus ostial dilation for surgery of the sinuses, including for the treatment of sinusitis. This procedure involves insertion of a balloon catheter device into a nasal sinus cavity to open blocked sinus ostia. 10/8/2013 SURG.00131 Lower Esophageal Sphincter Augmentation Devices for the Treatment of Gastroesophageal Reflux Disease (GERD) This policy addresses the use of non-endoscopic devices intended for the treatment of gastroesophageal reflux disease (GERD). 10/8/2013 SURG.00133 Alcohol Septal Ablation for Treatment of Hypertropic Cardiomyopathy This policy addresses alcohol septal ablation for the treatment of hypertrophic cardiomyopathy (HCM). HCM has also been referred to in the published literatu re as hypertrophic obstructive cardiomyopathy (HOCM). 10/8/2013 TRANS.00014 Mechanical Circulatory Assist Devices (Ventricular Assist Devices, Percutaneous Ventricular Assist Devices and Artificial Hearts) This policy addresses ventricular assist devices (VADs), percutaneous ventricular assist devices (pVADs) and artificial hearts. 10/8/2013 CG-MED-22 Neuropsychological Testing This guideline addresses the use of neuropsychological testing, also known as psychometric testing, which refers to a quantitative, comprehensive evaluation of cognitive, motor and behavioral functional abilities related to developmental, degenerative, and acquired brain disorders. 12/2/2013 CG-MED-46 Ambulatory Electroencephalography This guideline addresses ambulatory electroencephalography monitoring in the outpatient (home) setting. This test records continuous and prolonged electrical activity of the brain to assist in the evaluation and diagnosis of seizure disorders and epilepsy syndromes. November 2013 35 of 39 ANNUAL REVIEW TOPICS 10/8/2013 ADMIN.00004 Medical Necessity Criteria 10/8/2013 ADMIN.00005 Investigational Criteria 10/8/2013 ADMIN.00006 Review of Services for Benefit Determinations in the Absence of a Company Applicable Medical Policy or Clinical Utilization Management (UM) Guideline 10/8/2013 ANC.00006 Biomagnetic Therapy 10/8/2013 ANC.00007 Cosmetic and Reconstructive Services: Skin Related 10/8/2013 ANC.00008 Cosmetic and Reconstructive Services of the Head and Neck 10/8/2013 DME.00004 Electrical Bone Growth Stimulation 10/8/2013 DME.00024 Transtympanic Micropressure for the Treatment of Meniere's Disease 10/8/2013 DME.00030 Altered Auditory Feedback (AAF) Devices for the Treatment of Stuttering 10/8/2013 DRUG.00017 Hyaluronan Injections in Joints Other Than the Knee 10/8/2013 DRUG.00031 Subcutaneous Hormone Replacement Implants 10/8/2013 GENE.00002 Preimplantation Genetic Diagnosis Testing 10/8/2013 GENE.00015 Predictive Genetic Testing for Non-Malignant Diseases 10/8/2013 LAB.00011 Analysis of Proteomic Patterns 10/8/2013 LAB.00016 Fecal Analysis in the Diagnosis of Intestinal Dysbiosis 10/8/2013 MED.00081 Cognitive Rehabilitation 10/8/2013 MED.00090 Wireless Capsule for the Evaluation of Suspected Gastric and Intestinal Motility Disorders 10/8/2013 MED.00098 Hyperoxemic Reperfusion Therapy 10/8/2013 RAD.00019 Magnetic Source Imaging and Magnetoencephalography 10/8/2013 RAD.00023 Single Photon Emission Computed Tomography (SPECT) Scans for Noncardiovascular Indications 10/8/2013 RAD.00034 Dynamic Spinal Visualization (Including Digital Motion X -ray and Cineradiography/ Videofluoroscopy) November 2013 36 of 39 10/8/2013 RAD.00042 SPECT/CT Fusion Imaging 10/8/2013 RAD.00045 Cerebral Perfusion Imaging Using Computed Tomography 10/8/2013 RAD.00046 Cerebral Perfusion Studies using Diffusion and Perfusion Magnetic Resonance Imaging 10/8/2013 RAD.00060 Digital Breast Tomosynthesis 10/8/2013 SURG.00005 Partial Left Ventriculectomy 10/8/2013 SURG.00010 Treatments for Urinary Incontinence 10/8/2013 SURG.00020 Bone-Anchored Hearing Aids 10/8/2013 SURG.00023 Breast Procedures; including Reconstructive Surgery, Implants and Other Breast Procedures 10/8/2013 SURG.00048 Panniculectomy and Abdominoplasty 10/8/2013 SURG.00051 Hip Resurfacing 10/8/2013 SURG.00054 Endovascular/Endoluminal Repair of Aortic Aneurysms, Aortoiliac Disease, Aortic Dissection and Aortic Transection 10/8/2013 SURG.00059 Recombinant Human Bone Morphogenetic Protein 10/8/2013 SURG.00074 Nasal Surgery for the Treatment of Obstructive Sleep Apnea (OSA) and Snoring 10/8/2013 SURG.00076 Nerve Graft after Prostatectomy 10/8/2013 SURG.00084 Implantable Middle Ear Hearing Aids 10/8/2013 SURG.00085 Mastectomy for Gynecomastia 10/8/2013 SURG.00090 Radiofrequency and Pulsed Radiofrequency Neurolysis for Tr igeminal Neuralgia (TGN) 10/8/2013 SURG.00105 Bicompartmental Knee Arthroplasty 10/8/2013 SURG.00116 High Resolution Anoscopy Screening for Anal Intraepithelial Neoplasia (AIN) and Squamous Cell Cancer of the Anus 10/8/2013 SURG.00118 Bronchial Thermoplasty 10/8/2013 SURG.00125 Radiofrequency and Pulsed Radiofrequency Ablation of Trigger Point Pain 10/8/2013 SURG.00126 Ablation of Soft Tissue using Irreversible Electroporation (IRE) November 2013 37 of 39 10/8/2013 SURG.00132 Devices for Maintaining Sinus Ostial Patency Fol lowing Sinus Surgery 10/8/2013 SURG.00134 Interspinous Fixation Devices 10/8/2013 TRANS.00035 Mesenchymal Stem Cell Therapy For Orthopedic Indications 10/8/2013 CG-ANC-03 Acupuncture 10/8/2013 CG-BEH-01 Assessment for Pervasive Developmental Disorders 10/8/2013 CG-DME-03 Neuromuscular Stimulation in the Treatment of Muscle Atrophy 10/8/2013 CG-DME-04 Electrical Nerve Stimulation, Transcutaneous, Percutaneous 10/8/2013 CG-DME-05 Cervical Traction Devices for Home Use 10/8/2013 CG-DME-07 Augmentative and Alternative Communication (AAC) Devices/Speech Generating Devices (SGD) 10/8/2013 CG-DME-08 Infant Home Apnea Monitors 10/8/2013 CG-DME-15 Hospital Beds and Accessories 10/8/2013 CG-DRUG-03 Beta Interferons or Glatiramer Acetate for Treatment of Mul tiple Sclerosis 10/8/2013 CG-DRUG-07 Hepatitis C Pegylated Interferon Antiviral Therapy 10/8/2013 CG-DRUG-13 Hepatitis B Interferon Antiviral Therapy 10/8/2013 CG-DRUG-27 Clostridial Collagenase Histolyticum Injection 10/8/2013 CG-MED-05 Ketogenic Diet for Treatment of Intractable Seizures 10/8/2013 CG-MED-23 Home Health 10/8/2013 CG-MED-31 Skilled Nursing Facility Services 10/8/2013 CG-REHAB-03 Pulmonary Rehabilitation 10/8/2013 CG-REHAB-09 Acute Inpatient Rehabilitation 10/8/2013 CG-SURG-05 Maze Procedure 10/8/2013 CG-SURG-07 Vertical Expandable Prosthetic Titanium Rib (VEPTR) 10/8/2013 CG-SURG-08 Sacral Nerve Stimulation as a Treatment of Neurogenic Bladder Secondary to Spinal Cord Injury November 2013 38 of 39 10/8/2013 CG-SURG-11 Surgical Treatment for Dupuytren's Contracture 10/8/2013 CG-SURG-18 Septoplasty CODING UPDATES OF EXISTING MEDICAL POLICIES OR CLINICAL UM GUIDELINES EFFECTIVE 2/1/2014 SURG.00113 Artificial Retinal Devices November 2013 39 of 39