Chemistry Bonding & Geometry Practice Test
advertisement
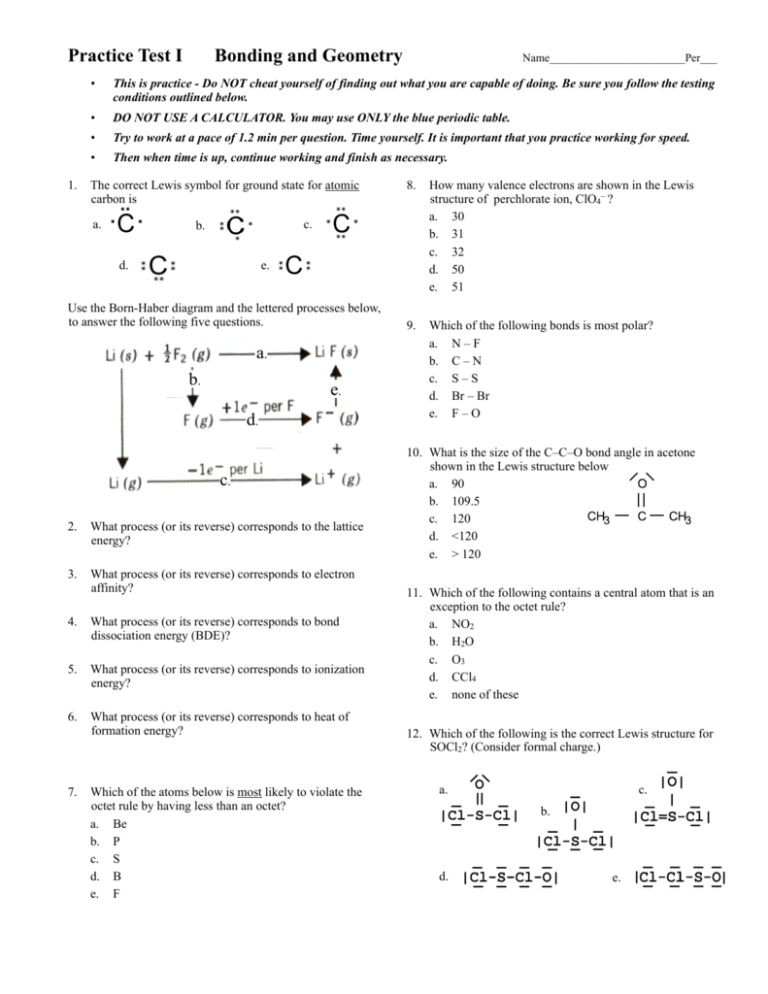
Practice Test I 1. Bonding and Geometry • This is practice - Do NOT cheat yourself of finding out what you are capable of doing. Be sure you follow the testing conditions outlined below. • DO NOT USE A CALCULATOR. You may use ONLY the blue periodic table. • Try to work at a pace of 1.2 min per question. Time yourself. It is important that you practice working for speed. • Then when time is up, continue working and finish as necessary. The correct Lewis symbol for ground state for atomic carbon is a. 8. How many valence electrons are shown in the Lewis structure of perchlorate ion, ClO4− ? a. 30 b. 31 c. 32 d. 50 e. 51 9. Which of the following bonds is most polar? a. N – F b. C – N c. S – S d. Br – Br e. F – O c. b. d. e. Use the Born-Haber diagram and the lettered processes below, to answer the following five questions. a. b. e. d. c. 2. What process (or its reverse) corresponds to the lattice energy? 3. What process (or its reverse) corresponds to electron affinity? 4. What process (or its reverse) corresponds to bond dissociation energy (BDE)? 5. What process (or its reverse) corresponds to ionization energy? 6. What process (or its reverse) corresponds to heat of formation energy? 7. Name________________________Per___ Which of the atoms below is most likely to violate the octet rule by having less than an octet? a. Be b. P c. S d. B e. F 10. What is the size of the C–C–O bond angle in acetone shown in the Lewis structure below a. 90 b. 109.5 c. 120 d. <120 e. > 120 11. Which of the following contains a central atom that is an exception to the octet rule? a. NO2 b. H2O c. O3 d. CCl4 e. none of these 12. Which of the following is the correct Lewis structure for SOCl2? (Consider formal charge.) a. c. b. d. e. MC Practice Test I Bonding & Geometry 13. Which one of the following molecules is a polar molecule? a. Cl2 b. CO2 c. NF3 d. CCl4 e. H2S 14. Which of the following molecular shapes has six atoms joined to a central atom? a. octahedral b. linear c. trigonal bipyramidal d. tetrahedral e. planar triangular 15. Which molecular shape has bond angles which are not all the same? a. linear b. planar triangular c. tetrahedral d. trigonal bipyramidal e. octahedral 16. What is the geometry of the electron domains in the molecule XeF2 a. linear b. planar triangular c. tetrahedral d. trigonal bipyramidal e. octahedral 17. The molecule BrF3 has how many lone pairs of electrons on the central atom? a. 0 b. 1 c. 2 d. 3 e. 4 18. What is the geometrical arrangement of electron domains in H2O? a. linear b. trigonal bipyramidal c. bent d. tetrahedral e. octahedral page 2 of 12 19. What is the shape of BrI3? a. square planar b. pyramidal c. T-shaped d. bent e. distorted tetrahedral 20. What type of hybridization is associated with a square planar molecular shape? a. sp b. sp2 c. sp3 d. sp3d e. sp3d2 21. What is the shape of the IF4− ion? a. square planar b. octahedral c. tetrahedral d. T-shaped e. square pyramidal 22. Which of the following is a polar species? a. CO2 b. PCl5 c. ICl2− d. TeCl4 e. CCl4 23. Among those listed below, which element will have the strongest tendency to form double bonds? a. Br b. B c. F d. O e. Mg 24. Which hybridization is associated with 3 domains? a. sp b. sp2 c. sp3 d. sp3d e. sp3d2 25. The molecule SF4 has how many electron domains on the central atom? a. 2 b. 3 c. 4 d. 5 e. 6 MC Practice Test I Bonding & Geometry 26. What is the hybridization of Br in BrF5? a. sp b. sp2 c. sp3 d. sp3d e. sp3d2 27. What shape for electron pairs is associated with sp3 hybridization? a. linear b. tetrahedral c. square planar d. octahedral e. trigonal planar 28. What hybridization is predicted for phosphorus in the PCl3 molecule? a. sp b. sp2 c. sp3 d. sp3d e. sp3d2 29. A double bond contains ___ sigma bond(s) and ___ pi (π) bond(s). a. 0, 2 b. 1, 2 c. 2, 0 d. 1, 1 e. 0, 1 30. What is the smallest angle that exists between domains in an octahedral structure? a. 78.5° b. 90.0° c. 109.5° d. 120.0° e. 180.0° 31. Which of the following elements is most likely to display sp3d hybridization? a. oxygen b. carbon c. nitrogen d. boron e. phosphorus page 3 of 12 32. How many sigma (σ) and pi (π) bonds are in a carbon dioxide molecule? a. four σ and zero π b. two σ and four π c. three σ and two π d. one σ and three π e. two σ and two π 33. What is the hybridization of the oxygen atoms in CH3OH and CO2, respectively? a. sp3, sp3 b. sp2, sp2 c. sp3, sp2 d. sp3, sp e. sp2, sp3 34. All of the following species contain two π-bonds EXCEPT a. SCN− b. OCS c. CO d. NO− e. H2CCO 35. How many unshared pair of electrons on the central atom in the molecule, XeF2? a. 1 b. 2 c. 3 d. 4 e. 5 36. Which of the following are true about BF3? i. trigonal planar ii. one unshared pair of electrons on B iii. polar molecule a. i only b. i and ii only c. i and iii only d. ii and iii only e. i, ii, and iii 37. Consider the chemical reaction below. BF3 + NH3 → BF3NH3 During this chemical reaction, the geometry around the boron atom changes from a. trigonal pyramid to tetrahedral b. trigonal planar to tetrahedral c. trigonal planar to octahedral d. trigonal pyramid to trigonal bipyramidal e. actually its geometry doesn’t change at all MC Practice Test I Bonding & Geometry 38. The melting point of CaS is higher than that of KCl. Explanations for this observation include which of the following? I. Ca2+ is more positively charged than K+ II. S2− is more negatively charged than Cl− III. The S2− ion is smaller than the Cl− ion. IV. The Ca2+ ion is smaller than the K+ ion a. II only b. I, II, IV only c. III and IV only d. II and III only e. I, II, III, and IV 39. Types of hybridization exhibited by the three C atoms in propene, CH3CHCH2, include which of the following? I. sp a. b. c. d. e. II. sp2 III. sp3 I only III only I and II only II and III only I, II, and III 40. Of the following molecules, which has the largest dipole moment? a. NH3 b. CO2 c. OF2 d. H2O e. CF4 41. The molecular geometry of SF4 is a. see-saw b. t-shape c. trigonal bipyramidal d. octahedral e. tetrahedron 42. In the molecule shown with the formula AF4 which element could be in the central position? a. S b. O c. Xe d. P e. It could be either a or b page 4 of 12 43. In order to exhibit delocalized π bonding, a molecule must have a. at least two π bonds b. at least two resonance structures c. at least three σ bonds d. at least four atoms e. a and c are both true 44. In ozone, O3 the formal charge on the central atom is a. 0 b. +1 c. −1 d. +2 e. −2 45. The Lewis structure of HCN shows that ______ has _______ nonbonding electron pairs. a. C, 1 b. N, 1 c. H, 1 d. N, 2 e. C, 2 46. According to the VSEPR model, the progressive decrease in the bond angles in the series of molecules CH4, NH3 and H2O is best accounted for by the a. increasing strength of bonds b. decreasing size of the central atom c. increasing the electronegativity of the central atom d. increasing number of unshared pairs of electrons e. decreasing repulsion between hydrogen atoms 47. The hybridization of the carbon atom in methane, CH4 is a. sp b. sp2 c. sp3 d. sp4 e. sp3d 48. The central iodine atom in ICl4− has _______ unshared electron pairs and ______ bonded electron pairs. a. 3, 2 b. 3, 1 c. 1, 3 d. 1, 4 e. 2, 4 Bonding & Geometry (A) CO2 (B) H2O (C) CH4 (D) NH3 (E) None of the molecules above satisfy the statement. 49. The molecule whose central atom’s electron domains are not tetrahedral. 50. The molecule with only one double bond. 51. The molecule with the largest dipole moment. 52. The molecule that has trigonal pyramidal geometry. 65. How many single covalent bonds must a silicon atom form to have a complete octet of its valence electrons? a. 4 b. 3 c. 2 d. 1 e. 0 66. The bond angles around the atoms 1, 2, and 3 in the molecule below are approximately ______, _______, and _______ respectively. a. 90°, 90°, 90° H O b. 120°, 120°, 90° •• H−C− C −O − H c. 120°, 120°, 109.5° •• 2 d. 109.5°, 120°, 109.5° 3 1 H e. 109.5°, 90°, 120° 54. The molecule that exhibits resonance. 55. The molecule with at least one pi bond. 56. The molecule with no non-bonded electron pairs. For next 7 questions refer to the following molecules. (You might find it useful to sketch a structure for each one.) (A) CH2O (B) H2S (C) C2H2 (D) PH3 (E) None of the molecules above satisfy the statement. 57. The molecule with a double bond. − − 53. The molecule with at least one bond angle of 109.5° 64. What is the maximum number of double bonds that a single carbon atom can form? a. 4 b. 3 c. 2 d. 1 e. 0 •• For the next 8 questions refer to the following molecules. (You might find it useful to sketch a structure for each one.) page 5 of 12 =• • MC Practice Test I 67. The hybridized orbitals around the C atom marked #2 in the structure above are a. sp b. sp2 c. sp3 d. sp3d e. sp3d2 68. Of the following atoms, which can not accommodate more than an octet of electrons? a. P b. Xe c. N d. S e. I 58. The molecule for which isomers can be written. 59. The molecule that has trigonal planar geometry. 60. The molecule with bond angles of 107° 61. The molecule that is linear. 62. The molecule with more than one pi bond. 63. The molecule with no non-bonded electron pairs. 69. For which of the following molecules can a valid Lewis structure not be drawn without violation the octet rule? a. PO43− b. SiF4 c. CF4 d. SeF4 e. NF3 MC Practice Test I Bonding & Geometry page 6 of 12 70. The electron-domain geometry and molecular geometry of iodine trichloride are ______ and ______ respectively. a. trigonal planar, trigonal planar b. tetrahedral, trigonal pyramidal c. trigonal bipyramidal, T-shaped d. octahedral, trigonal planar e. T-shaped, trigonal planar 76. When counting domains, a triple bond a. Should not be counted as a domain b. Depends on the geometry as to how many domains it should be counted as c. Should be counted as three domains d. Should be counted as two domains due to the 2 π bonds e. Should be counted as only one domain 71. If the electron domain geometry of some sulfur-centered compound is trigonal bipyramidal, then the hybridization of the central sulfur atom must be _______. a. sp b. sp2 c. sp3 d. sp3d e. sp3d2 77. The electron-domain geometry and the molecular geometry of a molecule of the general formula ABx will be the same if a. there are no unshared electron pairs on the central atom b. there is more than one central atom c. x is greater than 4 d. x is less than 4 e. the octet rule is obeyed 72. How many unhybridized p atomic orbitals are on an sp hybridized carbon atom? a. 0 b. 1 c. 2 d. 3 e. 4 78. Which of the molecules below is nonpolar? a. BF3 b. NF3 c. IF3 d. PF3 e. BrF3 73. What would be the electron-domain geometry of a central atom for which the hybridization of orbitals are sp? a. octahedral b. linear c. trigonal planar d. trigonal bipyramidal e. tetrahedral 74. The shortest F−Xe−F bond angle in the XeF4 molecule would be approximately a. 60° b. 90° c. 109.5° d. 120° e. 180° 75. Which covalent bond is the longest a. single b. double c. triple d. they are all the same length e. nonpolar 79. What is the molecular geometry of the H3O+ ion? a. linear b. tetrahedral c. bent d. trigonal pyramidal e. trigonal planar Practice Test I Bonding and Geometry Free Response 80. Draw the Lewis structure for carbonate: CO32−. a. What is the shape of the electron domains around the central carbon? b. What is the shape of the carbonate ion? c. What are the bond angles in carbonate? d. Does the carbonate ion exhibit resonance? e. Does delocalization occur? What does this term mean? f. Comment on the bond lengths in carbonate. 81. Bond enthalpy values can be used to calculate and estimation for ∆Hrx when the ∆Hf° values are not available for all the compounds in the reaction. Use bond enthalpy values from the back of your ∆Hf tables to calculate the ∆Hrx for the reaction below. Do your work in the space below. Circle your final answer. Be sure and label it appropriately. H2CNH2 + H2O → CH2O + NH3 MC Practice Test I (pg 8 of 12) ANSWERS Bonding & Geometry 1. a Lewis structure will represent the valence electrons, thus you must realize that C is 2s2 2p2. The s2 electrons will paired and p2 electrons will be in separate orbitals. 2. e A Born-Haber diagram represents chemical reactions that work together. Essentially it is fancy method of showing Hess’ Law. It is used to determine the energy for the reaction of separating an ionic compound into its separated ions. This is called lattice energy. In the diagram shown, the arrow indicates the reverse of lattice energy. 3. d Remember that electron affinity is the reaction representing the process of a neutral atom taking in an electron to produce a negative ion. 4. b Bond dissociation energy is the energy needed to break a particular bond. 5. c Ionization energy is the energy required to convert a neutral atom into a positive ion. 6. a The definition of heat of formation is the reaction of elements in their standard form to produce one mole of a chemical compound. 7. d Boron is an anomaly in that it can produce fairly stable compounds with only three other atoms bonded to it with single bonds, providing only 6 valence electrons around it. 8. c 7 (Cl) + 4 × 6 (O) + 1 (extra electron) = 32 e− 9. a The further apart two atoms are on the periodic table, the greater the polarity of a bond between those two atoms, thus N and F meet this criteria. 10. e The central carbon has 3 domains (a double bond is indeed two bonds, 4 electrons, but only counted as one domain). Three domains always results in trigonal pyramid with has bond angles of 120º. The pi bond of the double bond (the “hot dog bun” bond) bulges resulting in a distorted trigonal planar structure. This bulge forces the other two electron pairs away, resulting in a C−C−O bond that is slightly larger that 120º. The C−C−C bond would be slightly less than 120º. 11. a An octet rule, is the rule that says each atom is bonding with other atoms in an attempt to achieve the stable configuration of 8 valence electrons. The NO2 molecule can not achieve this because it has an odd number of valence electrons. The least electronegative atom will come up short with only 7 valence electrons around it. 12. b The SOCl2 molecule has 26 valence electrons, thus we need 13 lines. Structure (a) can be ruled out because it has only 12 valence pairs. Structure (c) can be ruled out because the less chlorine has more than an octet, and we only ever put expanded octets on central atoms. The remaining structures all have formal charge, thus we must choose the structure with the lowest and least amount of formal charge, while placing the negative formal charge on the most electronegative atom, and the positive formal charge on the least electronegative atom. If you do not know how to count/apply formal charge, please read the back of NS I.1 or seek extra help. 13. c Determining the polarity of a molecule is a two part process. First you must establish if there are any polar bonds, then you must consider the arrangement of those bonds. If the polar bonds are asymmetrically arranged, the polar bonds will not cancel out, and the molecule will be polar. The highly polar N−F bonds, together with the trigonal pyramid structure of the molecule will result in a polar molecule. 14. a This is a memorize − the last structure below has six atoms attached. 2 1 2 2 1 3 4 4 1 4 3 1 3 3 1 2 2 5 5 6 15. d Referring to the diagram above, you can see that trigonal bipyramid has equatorial atoms, 120º apart from each other, and has axial atoms, only 90º apart from the equatorial atoms. 16. d First you must add the valance electrons and then draw a Lewis structure. The molecule has 22 valence electrons give rise to the 5 domains are trigonal bipyramid, the 3 unshared pairs which always locate into the axial position making the geometry of the molecule linear. 17. c Again, there is no substitute for being able to draw a correct Lewis Structure. BrF3 has 28 valence electrons resulting in the Lewis structure shown. The five electron domains are trigonal bipyramid. F Xe F I Br I I MC Practice Test I (pg 9 of 12) Bonding & Geometry ANSWERS 18. d An accurate Lewis structure with 8 valence electrons results in the Lewis structure shown. Four electron domains around the central oxygen atom is tetrahedron. 19. c Again, an accurate Lewis structure is essential. This is the same molecule as in question #17. The trigonal bipyramid set of domains with two unshared pair of electrons on the central bromine atom results in a T-shaped molecule. 20. e 21. a 22. d I Br I Square planar shape molecule arises from an six electron domains which is an octahedral set of electron domains. Six electron domains must be built from six orbitals, thus sp3d2. − An accurate Lewis structure drawn with 36 valence electrons as shown, results in six domains around the central F atom, two of which are unshared pair. These two unshared pair will orient opposite to each other, resulting in square shape for the molecule. As outlined in the answer to #13, determining the polarity of a molecule Cl is a two part process. First you must establish if there are any polar bonds, then you must consider the arrangement of those bonds. Cl Te Cl Considering the arrangement requires the drawing of accurate Lewis Cl structures to be able to look for unshared pairs around the central atom. If the polar bonds are asymmetrically arranged, the polar bonds will not cancel out, and the molecule will be polar. All of these species have polar Cl I bonds, though only the TeCl4 molecule, which has five domains with one unshared pair is unsymmetrically arranged in the see saw shape. ⎡ ⎢ ⎣ ⎡ ⎢ ⎣ I I F F I F F Cl Cl C Cl Cl Cl 23. d Oxygen with its s2p4 electron configuration most often forms two bonds, either two single bonds or one double bond. Boron is able to form 3 or 4 bonds, but rarely double bonds. Fluorine will NEVER form double bonds. Magnesium makes ionic bonds, and lastly bromine with it’s 7 valence electrons is far more likely to engage only in single bonding. 24. b 25. d Three domains means 3 orbitals must have been used to make the hybrid orbitals that go into building those 3 domains, thus sp2 hybridization produces those 3 domains. #26 There is no substitute for writing an accurate Lewis structure. 26. e Draw a Lewis structure to see the six domains which means six orbitals must be used to make those domains. 27. b sp3 hybridization means 4 orbitals, thus 4 domains, thus tetrahedron. 28. c PCl3 has 26 valence electrons resulting in the Lewis structure shown. There are four domains, thus sp3 hybridization. 29. d All single bonds are sigma bonds. Double bonds are made of a sigma and a pi bond. Consider the sigma and pi bond in the C=C bond in ethene. 30. b The octahedral structure has six domains. See the diagram to the right. 31. e In order for d orbitals to be included in the hybridization of an atom, the atom must be in the third period or higher, since elements in the second period do not have the capacity to have d orbitals. 32. e Two double bonds must be 2 pi and 2 sigma bonds. 33. c As indicated by the structure to the right, the oxygen in CH3OH has four domains, indicating sp3 hybridization, but the three domains around either oxygen in CO2 as drawn above, indicates sp2 hybridization. 34. d SCN− has 16 valence electrons, OCS has 16 valence electrons, CO has 10 valence electrons, NO− has 12 valence electrons, H2CCO has 16 valence electrons. Draw the Lewis structures as shown to the right, and observe that only NO− has a single pi bond. 35. c The XeF2 molecule has 22 valence electrons, resulting in 3 unshared pairs on the central Xe atom with its expanded octet. #27 #25 #30 MC Practice Test I (pg 10 of 12) Bonding & Geometry ANSWERS 36. a BF3 is an exception to the octet rule and is a molecule that can exist with only six valence electrons, resulting in a trigonal planar structure. The symmetrically opposed B−F bonds will result in a nonpolar molecule. It will not have an unshared pair on the boron atom. 37. b Observe the changing geometry of the boron atom as it participates in the Lewis acid base reaction shown in the photographs of the model kits. Boron starts with three domains (trigonal planar) and ends with four domains (tetrahedral). H N : H H H F B F F b 39. d First you must draw the molecule propene. Pro− means three carbon chain, −ene means double bond. Thus the molecule has the structure shown to the right. The carbon on the left has four domains which would be sp3 hybridization, and the carbon in the middle and on the right each have three domains and are both sp2 hybridized. 40. d All of the bonds in these five molecules are polar, this means that you must draw all five Lewis structures to consider the symmetry or lack thereof of each of the structures. important to draw all five. From this you should be able to notice that in the CO2 and CF4 molecules, the polar bonds cancel out leaving those two molecules as nonpolar. For the remaining three molecules, the presence of the unshared pairs on the central atom make the molecule polar. Thus we need to go back to the periodic table and consider the electronegativity of the atoms involved to determine that the H−O bond is probably more polar than the O−F or the N−H bonds. a N F H F Lattice energy is a number that tells us the energy H required to convert an ionic compound into its individual gaseous atoms. The larger the lattice energy, the stronger the ionic bond. The strength of ionic bonds is influenced by two factors. First and foremost, the magnitude of the ionic charges, this of course makes the Ca2+S2− ionic bond stronger than the K+Cl− ionic bond. Further, the Ca2+ ion is smaller than the K+ ion, since these two ions are isoelectronic with 18 electrons each, however the calcium ion has 20 protons, while the potassium ion has only 19 protons. 38. 41. F B The SeF4 molecule has 34 valence electrons resulting in the Lewis structure shown. The five domains on the central Se atom, with one of the domains as an unshared pair, result in see saw shape. O Se C F Se Se F F F 42. a If the molecule shown has 4 F’s, that would be 28 electrons, and in order to end up with the unshared pair on the central atom, we would need 6 more valence electrons. This would make you think either S or O would get the job done, however, since the central atom is an expanded octet, the central atom must be in the third period of the periodic table so that is an atom that has d orbitals. 43. b Delocalized pi bonding occurs when a molecule exhibits resonance, and in order to exhibit resonance, a molecule must have a double bond, and be able to exhibit at least two valid resonance structures. Please remember that not all structures with a double bond exhibit resonance structures, and sometimes three resonance structures can be drawn. 44. b First draw an accurate Lewis structure for ozone with its 18 valance electrons. Then apply formal charge. The oxygen in the middle is “assigned” 5 electrons, compared to the 6 valence electrons in atomic oxygen, thus it is assigned a formal charge of +1. The oxygen on the right would be assigned a formal charge of −1. +1 −1 45. b The HCN molecule has 10 valence electrons resulting in the Lewis structure shown to the right. Thus you can see the only nonbonding pairs reside on the nitrogen atom. Remember that the formal charge will be bad is you put nitrogen in the center of this molecule. It is always good to put carbon in the “middle” of the molecules that you draw, this way the carbon is unlikely to end up with unshared pairs which leads to formal charge. 46. d Each of these three molecules have 8 valence electrons. This changes the number of unshared pairs on the central atom increases. Unshared pairs of electrons are held in place by only one nucleus, not two, and thus take up more room, resulting in greater repulsion on the bonded pairs of electrons and distorting the tetrahedron. 47. c The Lewis structure of the methane molecule shown in the previous problem clearly has four domains on the central atom, requiring three valence orbitals, resulting in sp3 hybridization. MC Practice Test I (pg 11 of 12) 48. e Bonding & Geometry ANSWERS The ICl− ion has 36 valence electrons, resulting in 18 e− pairs. The correct Lewis structure is shown to the right. ⎡ ⎢ ⎣ Cl ⎡− ⎢ ⎣ Cl I Cl 49. a For #49−56, it would be best to draw the Lewis structures for all four molecules. I have shown them to the right. CO2 has on two domains on the central atom resulting in a linear shape, not tetrahedral. 50. e Since CO2 has two double bonds, and none of the remaining 3 have no double bonds, thus you must choose (e) none of the above. 51. b The molecule with the largest dipole moment is the same as the most polar. CO2 has polar bonds, but is linear and is thus nonpolar. CH4 is nonpolar since its bonds are essentially nonpolars, and it has a symmetrical shape anyway. If NH3 and H2O, the H−O bonds are more polar than the N−H bonds, resulting in a more polar molecule. 52. d The four domains with one unshared pair in the NH3 molecule make it trigonal pyramid in shape. 53. c Remember that the unshared pairs on H2O and NH3 cause the tetrahedral set of domains to be distorted. Thus one unshared pair of electrons in the NH3 molecule causes the H−N−H bond to be 107º and the two pair of non-bonded electrons in the water molecule cause the H−O−H bond to be 105º. The O=C=O bond in linear CO2 is obviously 180º, thus the CH4 molecule is the one in which the H−C−H bond is 109.5º 54. e None of these molecules exhibit resonance. Only the CO2 has double bonds, but no other structure in which the double bonded have been shifted can be written. You could conceivable draw a CO2 structure with a triple and single bond, however, there would be formal charge on this molecule and thus making it not valid, since there is no formal charge on the double bonded CO2 molecule. 55. a Remember that every double bond is made of one sigma and one pi bond, thus the CO2 molecule has two sigma and two pi bonds. 56. c While the CO2 molecule has no non-bonded pairs on the central atom, it does have non-bonded pairs on the oxygen atoms. Only the CH4 molecule has no non-bonded pairs on any of its atoms. 57. a For #57−63, it would be best to draw the Lewis structures for all four molecules. I have shown them to the right. Only formaldehyde, CH2O has a double bond. 58. e No isomers can be drawn for any of these simple molecules. 59. a Three domains produce trigonal planar geometry. This happens around the central carbon in the CH2O molecule. 60. d Ethyne, C2H2, with it’s triple bond, has linear geometry around both carbon atoms. 61. c The one unshared pair of electrons in the NH3 molecule causes the tetrahedron of domains around the nitrogen to be distorted, resulting in the the H−N−H bond to be 107º. 62. c The triple bond in ethyne, C2H2, is made of one sigma bond and two pi bonds. 63. c As you can see it is ethyne, C2H2, which has no non-bonded pairs of electrons. 64. c As in the carbon dioxide molecule, the carbon could form two double bonds. 65. a Silicon (like carbon) with it’s four valence electrons can connect with four single bonds to complete its octet. 66. d In the structure shown, carbon 1 has four domains, bond angle 109.5º, carbon 2 has three domains, bond angle of 120º, and oxygen 3 has four domains, with two of the pairs are unshared, resulting in a bond angle of ~109.5º (105º). 67. b Three domains will always be sp2 hydbridization. 68. c Atoms in the first or second period, without any d orbitals, can not accommodate an expanded octet. 69. d The SeF4 molecule has 34 valence electrons, and must be drawn with an expanded octet on the central Se atom. 70. c Again, there is no substitute for drawing a Lewis structure for this molecule that has 28 valence electrons. Remember that the unshared pairs in a trigonal bipyramid set of domains always are in the equatorial position. sp3d 71. d Trigonal bipyramid is five domains, which must be hybridization. 72. c If you have an sp hybridized carbon atom, the remaining 2 p orbitals must be unhybridized. 73. b sp hybridization is always linear in shape. 74. b Draw a Lewis structure to see the Xe central atom has an octahedral set of domains which will result in 90º angles. Cl I Se Cl Cl Cl Cl I Cl Cl MC Practice Test I (pg 12 of 12) Bonding & Geometry ANSWERS 75. a For bonds made of the same two atoms, C−C, C=C, and C≡C, the single bond will be longest. The double bonds are shorter because the unhybridized p orbitals must be closer together for them to overlap and make the bond. 76. e A domain is an electron group oriented in the same general direction, thus a single domain can be a single bond, a double bond (one domain), a triple bond (one domain), or a non-bonded pair. 77. a No unshared pairs on the central atom mean that domain geometry and molecular geometry are the same. 78. a Non-polarity will result if the polar bonds in the molecule − all of the bonds in the listed molecules are polar − arranged in a non-symmetrical arrangement. No unshared pairs of electrons on the central atom will lead to non-symmetrical arrangement. Only the BF3 molecule has a symmetrical − trigonal planar − arrangement. I P 79. d Draw a Lewis structure to see that there is a tetrahedron of domains around the central oxygen atom. 80. The carbonate ion has three resonance structures. a. trigonal planar b. trigonal planar since there are no unshared pairs on the central carbon. c. Three domains are always 120º d. yes, the double bond can be in any of the three possible locations e. yes, the electrons are delocalizes across all three bonds. f. the bond lengths will all be the same, somewhere between single and double length (closer to single) 81. ∆H = −40 kJ • Drawing the Lewis structures will show a bond inventory will show • Bonds breaking: 1 × C=N (615), 2 × C−H(413), 1 × N−H(391), 2 × O−H (463) • Bonds forming: 1 × C=O (799), 2 × C−H(413), 3 × N−H (391) • Note that you can cancel out 1 × N−H and 2 × C−H from breaking and forming for the net result of −40 kJ








