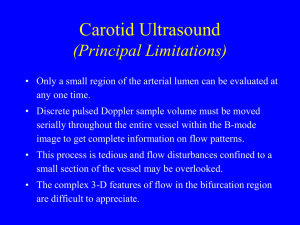
Peripheral chemoreceptors determine the respiratory sensitivity of

J Physiol 588.13 (2010) pp 2455–2471
Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO
2
Gregory M. Blain
1
,
2
, Curtis A. Smith
1
, Kathleen S. Henderson
1
and Jerome A. Dempsey
1
1 The John Rankin Laboratory of Pulmonary Medicine, Department of Population Health Sciences, School of Medicine and Public Health,
University of Wisconsin, Madison, WI, USA
2 LEMH – EA 4488, Faculty of Sport Sciences, University of Lille 2, Ronchin, France
We assessed the contribution of carotid body chemoreceptors to the ventilatory response to specific CNS hypercapnia in eight unanaesthetized, awake dogs. We denervated one carotid body (CB) and used extracorporeal blood perfusion of the reversibly isolated remaining
CB to maintain normal CB blood gases (normoxic, normocapnic perfusate), to inhibit
(hyperoxic, hypocapnic perfusate) or to stimulate (hypoxic, normocapnic perfusate) the CB chemoreflex, while the systemic circulation, and therefore the CNS and central chemoreceptors, were exposed consecutively to four progressive levels of systemic arterial hypercapnia via increased fractional inspired CO
2 for 7 min at each level. Neither unilateral CB denervation nor CB perfusion, per se
, affected breathing. Relative to CB control conditions (normoxic, normocapnic perfusion), we found that CB chemoreflex inhibition decreased the slope of the ventilatory response to CNS hypercapnia in all dogs to an average of 19% of control values (range 0–38%; n = 6), whereas CB chemoreflex stimulation increased the slope of the ventilatory response to CNS hypercapnia in all dogs to an average of 223% of control values
(range 204–235%; n = 4). We conclude that the gain of the CNS CO
2
/H + chemoreceptors in dogs is critically dependent on CB afferent activity and that CNS–CB interaction results in hyperadditive ventilatory responses to central hypercapnia.
(Received 8 January 2010; accepted after revision 23 April 2010; first published online 26 April 2010)
Corresponding author G. M. Blain: LEMH – EA 4488, Facult´e des Sciences du Sport, Universit´e de Lille 2, 9 rue de l’Universit´e, 59 790 Ronchin, France. Email: gregoryblain@ymail.com
Abbreviations CB, carotid body; CBX, carotid body denervation; EMG di
, diaphragm electromyogram; F
ICO2
, fractional inspired CO
2 arterial P
CO2
;
; LPBN, lateral parabrachial nucleus; MAP, mean arterial pressure; NTS, nucleus tractus solitarii;
P
CBO2
, carotid body P
O2
; P
CBCO2
, carotid body P
CO2
; P
ETCO2
, end-tidal P
CO2
P aCO2
; RTN, retrotrapezoid nucleus;
,
T
E
, duration of expiration; T
I
; duration of inspiration; V
T
, tidal volume.
2455
Introduction
We have known for more than a century that the levels of CO
2 in the systemic circulation and in the brain are the major determinants of breathing in humans and other mammals during wakefulness at rest and especially during sleep (Haldane & Priestly, 1905; Skatrud
& Dempsey, 1983). Subsequently, important sites of CO
2 chemoreception were discovered in the peripheral circulation, chiefly the carotid body (CB) chemoreceptors
(Heymans et al.
1930), and in the medulla oblongata
(central chemoreceptors; Loeschcke et al.
1963; Nattie
& Li, 2009). It was generally believed that each of these sets of chemoreceptors influenced breathing only by responding independently to chemical changes in their immediate environment (Loeschcke et al.
1963;
Fencl et al.
1966). Accordingly, much attention has been devoted to comparing and contrasting the relative contributions of peripheral versus central chemoreceptors to the overall ventilatory response to hypercapnia and to the changes and/or interindividual differences in ventilatory control in health and disease (Heymans et al.
1930; Comroe & Schmidt, 1938; Mitchell et al.
1963; Fencl et al.
1966; Dempsey & Forster, 1982).
However, it has been recognized for many years that if central–peripheral interaction is other than additive, modulation of one set of chemoreceptors by the other is highly likely (e.g. Adams & Severns, 1982).
Recent neuroanatomical and neurophysiological findings have revealed that CB afferents converge on putative chemosensitive/integrating neurons in the medulla; excitatory output from these neurons then impinges on
2010 The Authors. Journal compilation C 2010 The Physiological Society DOI: 10.1113/jphysiol.2010.187211
2456 G. M. Blain and others medullary respiratory rhythm/pattern-generating centres
(Stornetta et al.
2006; Takakura et al.
2006; Guyenet et al.
2008 a , b ; Zhang et al.
2009). This neural circuitry is consistent with predictions of modulation of central chemoreceptors by CB afferent inputs (Guyenet et al.
2008 b ).
Previous attempts to define peripheral–central chemoreceptor interactions, using such techniques as separate perfusions of medullary and/or carotid sinus regions, temporal separation of ventilatory responses in the intact animal or human and bilateral carotid chemoreceptor denervation, have yielded conflicting results. Most reports claim hypoadditive effects of peripheral inputs on the central CO
2 of central CO
2
/H + response and/or stimulation/inhibition on peripheral chemosensitivity (Gesell et al.
1940; Kiwull et al.
1976;
Giese et al.
1978; Eldridge et al.
1981; Smith et al.
1984;
Day & Wilson, 2007, 2009). Others have shown purely additive effects between the chemoreceptors (Heeringa et al.
1979; Schlaefke et al.
1979; van Beek et al.
1983;
Daristotle & Bisgard, 1989; Clement et al.
1992; Dahan et al.
1994; Clement et al.
1995; St Croix et al.
1996), with a few reports of hyperadditive interactive effects (Loeschcke et al.
1963; Mitchell, 1965; Lahiri & DeLaney, 1975 b ;
Robbins, 1988). Limitations to these findings include the marked alterations in control system sensitivities induced by decerebration, anaesthesia and/or sensory denervations
(Heeringa et al.
1980; Musch et al.
1980; Hayashi & Sinclair,
1991), a limited range of stimuli applied to either or both sets of chemoreceptors (Daristotle & Bisgard, 1989) and/or a lack of complete functional separation of the two sets of chemoreceptors in the intact preparation because of the effects of post-stimulus short-term potentiation on ventilatory output (Gesell & Hamilton, 1941; Dutton et al.
1967; Eldridge & Gill-Kumar, 1980; also see
Discussion).
Our goal was to assess the functional importance of this recently described model of circuitry connecting peripheral with central medullary chemoreceptors
(Guyenet, 2008; Guyenet et al.
2008 a ), through the use of an intact, unanaesthetized canine model that provides a separate perfusion of the isolated carotid chemoreceptors without alteration of the functional characteristics of the normal intact ventilatory control system. We show that physiological inhibition of the isolated CB chemoreceptor output eliminates a substantial amount of the normal central chemoreceptor ventilatory responsiveness to
CO
2
, whereas physiological stimulation of the CB chemoreceptor significantly enhances ventilatory responsiveness to central hypercapnia. These unique findings in the intact and unanaesthetized animal demonstrate the functional importance to breathing in this species of the peripheral–central chemoreceptor neural linkage and its synergistic interactions. If these findings hold true for other species, they could have broad implications for our understanding of ventilatory control.
Methods
Ethical approval
J Physiol 588.13
Experimental protocols and the roles of each investigator involved were approved by the Animal Care and Use
Committee of The University of Wisconsin-Madison
School of Medicine and Public Health and were performed with strict adherence to all American Association for the
Accreditation of Laboratory Animal Care International
(AAALAC) and National Institutes of Health guidelines embodied in the Guide for the Care and Use of Laboratory
Animals published by the US National Institutes of Health
(NIH publication no. 85-23, revised 1996). All authors have read the article by Drummond (2009).
Animals
Studies were performed during wakefulness on eight unanaesthetized, spayed, female, mixed-breed dogs
(20–25 kg). The dogs were trained to lie quietly in an air-conditioned (19–22 ◦ C), sound-attenuated chamber.
The dogs were housed in an AAALAC accredited animal care facility.
Surgical procedures and chronic instrumentation
Our preparation required two surgical procedures performed under general anaesthesia (pre-medicated with acepromazine (0.04–0.08 mg kg − 1
S .
C . or I .
M .) and atropine (0.02–0.044 mg kg − 1 propofol (3–5 mg kg − 1
I
S
.
C
. or
I
.
M
.); induced with
.
V
.); intubated immediately with a
7 mmID cuffed endotracheal tube and maintained with
1–2% isoflurane in O
2 with mechanical ventilation), with strict sterile surgical techniques and appropriate postoperative analgesics and antibiotics. No paralyzing agents were used. In the first procedure, using a ventral abdominal midline approach, dogs were subjected to ovariohysterectomy, a chronic indwelling catheter was placed into the abdominal aorta via a branch of the femoral artery, and bipolar electromyogram (EMG) recording electrodes were installed into the costal diaphragm. After a recovery interval of at least 3 weeks, a second procedure was performed in which the left CB was denervated
(CBX), and the right carotid sinus was equipped with a vascular occluder and catheter to permit extracorporeal perfusion of the reversibly isolated carotid sinus–carotid body. A chronic indwelling catheter was also placed in the abdominal vena cava via a branch of the femoral vein. Catheters were tunnelled subcutaneously to the cephalad portion of the dog’s back, where they were exteriorized. Dogs recovered for at least 4 days before study. The instrumentation was protected with a heavy
2010 The Authors. Journal compilation C 2010 The Physiological Society
J Physiol 588.13
Peripheral–central chemoreceptor interdependence nylon jacket and a padded ‘Elizabethan’ collar modified to allow normal eating and drinking.
Postoperative analgesia was buprenorphine (0.005–0.02 mg kg − 1
I achieved with
.
M
. or
I
.
V
. every
6–12 h; initial dose administered 30 min prior to the end of surgery) for the first 24 h in the first procedure and 12 h in the less invasive second procedure. We also administered one subcutaneous dose of carprofen (Pfizer,
NY, USA, 4 mg kg − 1 ) 1–2 h prior to the start of surgery.
We continued carprofen
P
.
O
. (2.2 mg kg − 1
4.4 mg kg − 1 every 24 h) for 3–7 days.
every 12 h or
A wide-spectrum oral antibiotic (cefalexin, Karalex
Pharma, NJ, USA, 20–30 mg kg − 1
P
.
O
. twice daily, or cefpodoxime proxetil (Pfizer, NY, USA), 10 mg kg − 1
P .
O .
once daily, or enrofloxacin (Bayer, KS, USA), 5–10 mg kg − 1
P .
O . once daily) was begun 24 h prior to surgery and was continued for 5–7 days postoperatively for the first procedure and 7–21 days for the second procedure. In addition, cefazolin (Sagent, IL, USA, 20–30 mg kg − 1 , slow
I
.
V
. injection) was administered immediately pre-operatively and every 2 h intra-operatively.
It is important to note that we used spayed female dogs to avoid problems with ventilatory effects of fluctuating ovarian hormone levels. We do not think this introduces a significant bias; we have discussed this in detail in a previous publication (Blain et al.
2009).
2457 training prior to study. Costal diaphragm EMG (EMG di
) signals were amplified, bandpass filtered (100–1000
Hz), rectified and moving-time-averaged (BMA-931;
MA-821RSP, CWE Ardmore, PA, USA). End-tidal P
P
CO
2
( P
ETCO
2
O
2 and
) were measured using a mass spectrometer
(MGA-1100, Perkin-Elmer, Waltham, MA, USA).
One millilitre arterial and perfusion circuit blood samples were analysed for pH, P
O
2 and P
CO
2 in a blood gas analyser (model ABL-505, Radiometer, Copenhagen,
Denmark). Blood pressure was recorded continuously from the femoral artery.
Ventilation and blood pressure signals were digitized
(128 Hz sampling frequency) and stored on the hard disk of a PC for subsequent analysis. Key signals were also recorded continuously on a polygraph (AstroMed
K2G, West Warwick, RI, USA). All ventilatory data were analysed on a breath-by-breath basis by means of custom analysis software developed in our laboratory.
Carotid sinus perfusion
Dogs lay unrestrained on a bed within the soundattenuated chamber. The extracorporeal circuit was primed with ∼ 700 ml of saline, 120 ml of allogenic blood (Animal Blood Resources International, Dixon, CA,
USA), and 2500 units of heparin and supplemented with
1000 units h − 1 . The P
CO
2
, P
O
2 and pH in the perfusion circuit were set by adjustment of the gas concentrations supplying the circuit and by addition of NaHCO
3
.
Complete isolation of the carotid body and absence of other peripheral chemosensitivity were confirmed by lack of ventilatory response to systemic injections of NaCN
(20–30
µ g kg − 1 ) during CB perfusion. The retrograde perfusion of the carotid sinus region raised blood pressure in the sinus by
<
10 mmHg, a level shown to have no effect on breathing in the unanaesthetized dog (Saupe et al.
1995). These techniques have been described in detail in previous publications (Smith et al.
1995, 2006; Curran et al.
2000); also see Fig. 1.
Experimental set-up and measurements
Ventilation was measured using a tight-fitting muzzle mask connected to a heated pneumotachograph (model
3700, Hans Rudolph, Kansas City, MO, USA) that was calibrated before each study with four known flows. The dogs were acclimated to the mask during several weeks of
Experimental protocol
Eupnoeic ventilation and blood gases and the ventilatory response to hypercapnia via increased fractional inspired
CO
2
( F
ICO
2
) were determined in three sets of conditions, as follows: (1) when the carotid bodies were both intact with normal endogenous CB perfusion; (2) after unilateral CB denervation with normal endogenous CB perfusion; and
(3) during normal, inhibitory or stimulatory CB perfusion
(see next paragraph). In the steady state of each set of CB conditions, the ventilatory response to CO
2 was assessed in a progressive, stepwise fashion consisting of 7 min intervals of air breathing followed by four increasingly higher levels of F
ICO
2 such that P
ETCO
2 was raised in 2–3 mmHg steps to achieve a maximal P
ETCO
2 of
10–12 mmHg above eupnoea. Ventilation, diaphragmatic
EMG activity, blood gases and blood pressure were obtained in the 5–7 min interval in each set of conditions.
For CB perfusion, each test protocol consisted of a
5 min control period (eupnoea), during which perfusion of the CB was endogenous, i.e. systemic arterial blood.
Two 1 ml blood samples were collected at ∼ 3 min for determination of blood gases and pH control values. Then,
CB perfusion was abruptly switched to the extracorporeal circuit (
<
2 s). The dogs were perfused in random order from the extracorporeal circuit with blood gases and pH concentrations matching a given dog’s eupnoeic values
(CB normal), or with hyperoxic ( P
O
2 and hypocapnic ( P
CO
2
>
500 mmHg)
∼ 20 mmHg) blood gases (CB inhibition), or with hypoxic ( P
O
2
∼ 40 mmHg) and normocapnic perfusate (CB stimulation). In the steady state of each set of CB perfusion conditions, the ventilatory response to central hypercapnia was assessed as described in the previous paragraph.
Once established, the isolated CB perfusion preparation usually remained functional for 5–7 days of
2010 The Authors. Journal compilation C 2010 The Physiological Society
2458 G. M. Blain and others experimentation. We devoted 2 or 3 days to repeating central hypercapnic response tests in each set of CB perfusate conditions in order to obtain several (usually
6–10) steady-state elevations in arterial P
CO
2 facilitate accurate quantification of central CO
2
( P aCO
2
) to response slopes. Accordingly, we were able to complete central CO
2 response slopes in all eight animals with normocapnic, normoxic CB perfusion and of these eight, we had sufficient time to complete CB inhibition in six and CB stimulation in four.
Upon completion of the protocol, dogs were humanely killed with an overdose of 200 mg propofol into the implanted
I
.
V
. catheter followed by 5 ml Beuthanasia-D
Special (Schering Plough, NJ, USA) via the same catheter after full unconsciousness was achieved.
Prior to killing, and under general isoflurane anaesthesia as described above under “Surgical Procedures
. . .
”, a terminal study was performed in one dog to measure blood flow in the brachiocephalic artery and the ascending aorta (i.e. cardiac output). Ultrasonic blood flow probes
(Transonic Systems, Ithaca, NY, USA) of appropriate size
J Physiol 588.13
were placed around each artery and blood flows measured for at least 5 min (see Miller et al.
2006 for detailed methods).
Statistics
Slopes of the CO
2 responses were determined by linear regression. Normality of the mean data was confirmed using the Shapiro–Wilk test. Significance of the differences in mean ventilatory response data between CB perfusion conditions was determined by means of Student’s paired t tests with Bonferoni correction for multiple comparisons. Differences were considered significant if
P
<
0.05.
Results
Effects of unilateral CB denervation and CB perfusion
Table 1 shows (column 2 CB versus 1 CB) that unilateral CBX did not change air-breathing
Figure 1. Schematic diagram showing the essential features of the isolated carotid body perfusion technique in the unanaesthetized dog (not to scale)
The aortic arch is just visible at the bottom of the diagram. The left carotid body is denervated, but blood supply to the CNS is left intact. On the right side the carotid body is left intact, but all branch arteries in the carotid sinus region are ligated except the lingual. Vascular casts of the carotid sinus region with these ligations (or occlusions in the case of the lingual artery) showed that the carotid sinus region was isolated (Smith et al.
1995). A perfusion catheter is placed in the (ligated) external carotid with its tip placed just caudal to the carotid sinus. Ligations are made distal to any vessels supplying the carotid body. An occluder is placed around the lingual artery such that it can be inflated to isolate the region during perfusion; between perfusions the occluder is deflated, allowing flow through the region to maintain patency. Thus, during perfusion, flow through the isolated carotid sinus region is retrograde at a pressure slightly ( ∼ 10 mmHg) higher than systemic; the flow rate is
40–60 ml min − 1
<
100 ml min − 1 (usually
), and excess perfused blood enters and mixes with the systemic circulation at the brachiocephalic artery, where we measured the flow rate as approximately 500 ml min − 1 (see Methods). Some of this admixture may reach the CNS via the right vertebral artery (see ‘ Limitations of our preparation ’ in Discussion for details).
Abbreviations: BA, brachiocephalic artery; CB, carotid body; CBX, carotid body denervation; CCA, common carotid artery; and VA, vertebral artery. (This figure is a modification of a figure published by Smith et al.
(1995).)
2010 The Authors. Journal compilation C 2010 The Physiological Society
J Physiol 588.13
Peripheral–central chemoreceptor interdependence 2459
Table 1. Eupnoeic values during air breathing for arterial blood gases, ventilation, EMG di and mean arterial pressure, showing effects of unilateral CB denervation, CB perfusion with normoxic normocapnia, and CB inhibition and stimulation
CB isolated and perfused
Arterial pH
Arterial P
CO
2
(mmHg)
Arterial P
O
2
(mmHg)
Minute ventilation (l min − 1 )
Tidal volume (l)
Breathing frequency
(breaths min − 1 )
EMG di
(a.u.)
MAP (mmHg)
7
.
39
34
100
4
0
.
.
12
(
.
.
12
35
.
2 CB n
9
9
3
=
±
±
±
±
±
±
8)
0
2
7
0
0
3
.
.
.
.
.
.
01
5
7
58
05
5
7
4
0
.
40
98
.
.
13
(
1 CB n
34
.
.
9
2
21
34
.
2
=
±
±
±
±
±
±
8)
0
2
7
0
0
2
.
.
.
.
.
.
02
7
3
96
05
9
CB normal
7
41
100
4
0
.
.
.
12
(
32
.
.
n
7
5
13
34
.
8
=
±
±
±
±
±
±
8)
0
2
8
0
0
2
.
.
.
.
.
.
03
6
5
67
06
8
CB inhibition
7
.
12
.
( n
27
8
=
±
±
6)
0
2
.
.
02
49
.
9 ± 5
.
2
7
∗
∗
99
.
0 ± 6
.
8
3
.
46 ± 0
.
91 ∗
0
.
27 ± 0
.
05 ∗
CB stimulation
( n = 4)
7
.
36 ± 0
.
03 ∗
36
.
4 ± 2
.
9 ∗
121
.
0 ± 19
.
2 ∗
4
.
62 ± 0
.
55 ∗
0
.
48 ± 0
.
02
10
.
1 ± 0
.
8
∗
0
.
68 ± 0
.
26
107
.
7 ± 9
.
0
0
.
67 ± 0
.
34
102
.
4 ± 7
.
4
0
.
63 ± 0
.
37
104
.
3 ± 10
.
7
0
.
42 ± 0
.
15 ∗
110
.
1 ± 19
.
7
0
.
76 ± 0
.
19
105
.
7 ± 6
.
7
Values are means ± S .
D . Abbreviations: 2 CB, the fully intact condition preceding unilateral carotid body (CB) denervation; 1 CB, the condition following unilateral CB denervation; CB normal, normoxic–normocapnic CB perfusion [carotid body and carotid body P
CO
2
( P
CBCO
2
P
) = 40 mmHg]; CB inhibition, hyperoxic–hypocapnic CB perfusion ( P
O
2
( P
CBO
2
CBO
2
) = 100 mmHg
>
500 mmHg and
P
CBCO
2
= 20 mmHg); CB stimulation, hypoxic–normocapnic CB perfusion ( P
CBO
2
= 40 mmHg and P
CBCO
2
= 40 mmHg); EMG di
, mean electrical activity of the costal diaphragm electromyogram in arbitrary units (a.u.); and MAP, mean arterial pressure. Note that during
CB inhibition, the inspired O
2 fraction was increased to maintain arterial P
O
2 at control values.
∗ Significant difference from CB normal,
P
<
0.05 (Student’s paired t test with Bonferoni correction for multiple comparisons).
spontaneous ventilation, EMG di
, arterial blood gases or mean arterial pressure, nor was the slope of the ventilatory response to increased
(2 CB 0.73
± 0.30 l min − 1 mmHg − 1
F
ICO
2 affected versus 1 CB 0.72
±
0.28 l min − 1 mmHg − 1 ; P
>
0.9). Thus, ventilatory control was identical to that in the fully intact animal. Comparison of columns 1 CB versus CB normal in Table 1 also shows that extracorporeal perfusion of the isolated CB in the intact animal with normoxic, normocapnic blood
(matched to prevailing spontaneous eupnoeic values in a given dog) had no significant effect on eupnoeic ventilation or arterial blood gases. These data document that ventilatory control characteristics in our awake, CB isolated and perfused animals were unchanged from those in the fully intact animals and that our CB perfusion and chemosensitivity testing methods were suitable for quantification of the effect of CB stimulation/inhibition on the ventilatory response to inspired and central CO
2
.
First, with CB inhibition (Fig. 2 A versus B ) there were significant reductions in air-breathing ventilation, tidal volume and EMG
(mean P aCO
2 di
, resulting in increased P aCO
2
+ 8.0 mmHg, range + 6.1 to + 12.5 mmHg).
Second, with CB stimulation (Fig. 2 C versus D ) there were significant increases in air-breathing ventilation, tidal volume and EMG
(mean P aCO
2 di
, resulting in decreased P aCO
2
− 7.9 mmHg, range − 6.7 to − 9.8 mmHg).
Third, progressive increases in breathing frequency, tidal volume and EMG di accompanied the increased levels of superimposed inspired, systemic and central hypercapnia during normal background conditions of CB normoxia plus normocapnia (Fig. 2 A and C ). Finally, these increases in breathing frequency, tidal volume and EMG di during specific central hypercapnia were clearly augmented in the face of background CB stimulation versus CB normal
(Fig. 2 C versus D ) and markedly reduced in the face of a background of CB inhibition versus CB normal (Fig. 2 A versus B ).
Effects of CB stimulation and inhibition on central
CO
2 response: polygraph recordings
Figure 2 shows polygraph recordings of typical experiments in two animals illustrating the effects of normal, inhibitory and stimulatory CB perfusion upon the ventilatory responses to air breathing (also see group mean values in columns CB normal, CB stimulation and CB inhibition of Table 1) and to four superimposed levels of central hypercapnia. Figure 2 B shows the effects of CB inhibition versus normal perfusion
(Fig. 2 A ) in one dog; similarly Fig. 2 D ) shows the effect of CB stimulation versus normal perfusion (Fig. 2 C ) in a second dog. We made the following observations.
Effects of CB stimulation and inhibition on central
CO
2 response: individual and group mean values
Quantification of the ventilatory response slopes for ventilatory components and diaphragmatic EMG to central hypercapnia against backgrounds of normal, stimulatory and inhibitory CB perfusion are shown for a typical animal in Fig. 3, and the ventilatory responses are shown for all individual dogs in Fig. 4. Group mean values for ventilatory components and diaphragmatic
EMG are shown in Fig. 5. Individual ventilatory and
EMG di response slope coefficients of all animals are given in Table 2. Note that each regression line defining the
2010 The Authors. Journal compilation C 2010 The Physiological Society
2460 G. M. Blain and others slope of the ventilatory response to central hypercapnia is based on multiple, steady-state levels of arterial P
CO
2 obtained during two or three test sessions for each type of
,
CB perfusate (normal, stimulation and inhibition) in each animal. In seven of the eight comparisons of the central
CO
2 response slopes amongst the varying conditions of
CB normal versus stimulation or inhibition, the stimulus levels (i.e.
P aCO
2
) overlapped by at least 5 mmHg.
All six animals subjected to CB chemoreceptor inhibition via hyperoxic hypocapnia showed a significant
J Physiol 588.13
and marked reduction in the slope of the central CO
2 ventilatory response, which averaged 19% (range 0–38%) of their respective response slopes during CB normoxic normocapnia. This reduced ventilatory response to central
CO
2 was due primarily to a reduced responsiveness of tidal volume (mean 29% of control; range 15–41%) associated with a reduction in EMG di
(mean 28% of control; range
24–37%) and to a lesser, but still significant, extent to a reduced response slope of breathing frequency (mean
39% of control; range − 70 to 222%). In turn, the reduced
Figure 2. Polygraph records of the effect of CB inhibition and stimulation on eupnoeic air-breathing ventilation and the ventilatory response to central hypercapnia
Representative segments of polygraph records of trials of control (normal endogenous CB perfusion) and extracorporeal CB perfusion with normal ( A and C ), inhibitory ( B ) and stimulatory perfusate blood gases ( D ) at the isolated CB.
A and B are from dog no. 3; C and D from dog no. 8. Dogs breathed air (segments 1 and 2) followed by step increases in inspired (and therefore arterial) of recorded data. Abbreviations: EMG arterial P
CO
2
; P
ETCO
2
, end-tidal P
CO
2 di
; and V
T
, tidal volume.
P
CO
2
(segments 3–6). Each segment represents 1 min
, moving-time-averaged electromyogram of the costal diaphragm; P aCO
2
,
2010 The Authors. Journal compilation C 2010 The Physiological Society
J Physiol 588.13
( T
I
).
central CO
2
Peripheral–central chemoreceptor interdependence response slope of breathing frequency (i.e. prolonged respiratory cycle length) to central CO
2 during CB chemoreceptor inhibition was attributable to a prolongation of the duration of expiration ( T
E
) relative to that with a normal CB (in 5 of 6 dogs), with no consistent change in the response slope for duration of inspiration
Conversely, all four animals exposed to stimulation of the isolated CB via hypoxic normocapnia showed a significant augmentation of their ventilatory response to which averaged 223% (range 204–235%) of their response slope during normal CB conditions. This increased ventilatory response slope was due mostly to an augmented response in breathing frequency (mean
239% of control; range 128–322%) and to a lesser, but still significant, extent to an increased response slope of tidal volume ( V
T
; mean 165% of control; range 117–264%), associated with an increase in EMG di
(mean 197% of control; range 131–250%). In turn, the augmented breathing frequency response to central CO
2 during CB chemoreceptor stimulation was due to a shortening of T
E relative to that with normal CB conditions (4 of 4 dogs), with no change in the T
I response slope.
A substantial, four- to fivefold, interindividual variation was found amongst the eight animals in the central response slopes to central hypercapnia in control conditions of CB normocapnic normoxia (range
0.3–1.4 l min − 1 mmHg − 1 ). All animals studied, regardless of the magnitudes of their CB normal CO
2 slopes, showed a reduced central CO
2 response responsiveness with
CB inhibition and an enhanced responsiveness with CB stimulation.
Discussion
Summary of findings
2461
Our study was concerned with defining the functional interactive effects of carotid chemoreceptor input on the ventilatory responsiveness of central chemoreceptors
Figure 3. A representative example (dog no. 1) of the ventilatory response to central hypercapnia with a background of normal, inhibitory and stimulatory CB perfusion
Minute ventilation ( A ), tidal volume ( B ), breathing frequency ( C ) and mean electrical activity of the costal diaphragm electromyogram ( D ) response slopes to step increases in arterial (and therefore central) P
CO
2 with a background of extracorporeal carotid body (CB) perfusion with normal (filled squares), inhibitory (open squares) and stimulatory perfusate blood gases (shaded triangles).
2010 The Authors. Journal compilation C 2010 The Physiological Society
2462 G. M. Blain and others J Physiol 588.13
2010 The Authors. Journal compilation C 2010 The Physiological Society
J Physiol 588.13
Peripheral–central chemoreceptor interdependence to hypercapnia. To this end, we employed an awake canine preparation with characteristics identical to those of the intact animal’s ventilatory control system, in which we determined the effects of altering the blood gases of the isolated, perfused carotid chemoreceptor, over a wide range of inhibition to stimulation, on the ventilatory responsiveness to superimposed steady-state central hypercapnia. We found marked, hyperadditive interactive effects between the chemoreceptors. Peripheral chemoreceptor stimulation (via CB hypoxia) more than doubled the slope of the central hypercapnic ventilatory response versus that obtained with a normocapnic normoxic carotid chemoreceptor.
Furthermore, peripheral chemoreceptor inhibition (via CB hyperoxic hypocapnia) reduced the average central CO
2 ventilatory response to about one-fifth of the control level. We discuss the implications of these characteristics of peripheral–central chemoreceptor synergism in terms of their limitations, their comparison with related findings in the literature and the new insights they might provide to explain unsolved problems of cardiorespiratory control in the intact animal and human.
Limitations of our preparation
Our technique does have the potential for some of the perfusate to reach the brain via the right vertebral artery after mixing with systemic blood in the brachiocephalic artery (Fig. 1). We do not think this is a significant limitation for the following reasons. Firstly, we measured the blood flow in the right brachiocephalic artery of an anaesthetized dog and found it to be about 15% of the cardiac output. In awake dogs of the same body mass and higher cardiac output than the anaesthetized animal
(Miller et al.
2006), we calculate the brachiocephalic flow in an unanaesthetized dog of ∼ 20–23 kg to be about
500 ml min − 1 . In our perfusion studies we infuse in a retrograde direction via the right carotid artery at an average rate of 40–60 ml min − 1 . Some of this blood flow will be diverted to the thyroid artery, which has no known anastomoses with vessels supplying the brain. The remaining flow will enter the brachiocephalic artery, where it will mix with systemic blood before the right vertebral artery branches off. Thus, there will be about a fiveto 10-fold dilution of the P
CO
2 in the perfusate blood that enters the brachiocephalic artery and is therefore available for the right vertebral artery. Importantly, the right vertebral artery is only one of three major arteries affect the CNS chemoreceptor at a given P aCO
2
2463 supplying the brain in our preparation; the left carotid and left vertebral arteries are intact and exit the aorta downstream from the branch point of the right brachiocephalic artery, thus eliminating the possibility of perfusate blood from reaching them. The systemic blood flowing in the left carotid and vertebral arteries would further dilute any contamination in the CNS from the right vertebral artery. Therefore, we believe that any contamination from perfusate into the brain would be very small and could not explain our findings. Secondly, the CB perfusate during the CB normal and CB stimulation perfusion conditions was normocapnic, so any contamination from the carotid sinus to the brainstem via the right vertebral artery would in an identical way in both the CB normal and CB stimulation conditions. While this might result in a very small offset, it should not affect the ventilatory response slopes to central hypercapnia. Thus, the conclusion of hyperadditive interaction would still be supported for these two conditions. Thirdly, a significant contamination during hypocapnic perfusion of the carotid sinus region seems unlikely for the following reasons: (1) during air breathing, we have shown previously (Blain et al.
2009) that carotid body hyperoxic and hypocapnic perfusion causes a decrease in ventilation that is 60% complete within one breath from the onset of CB perfusion and 90% complete within two breaths, thus speaking to the importance of carotid body hyperoxic hypocapnia alone as the primary, if not sole, determinant of the inhibition of ventilation noted in the present study; and (2) any small dilution of the central P
CO
2 during systemic hypocapnia would be expected to affect the position but not the slopes of the central ventilatory response to CO
2
, which were reduced by 80% with carotid body hyperoxic hypocapnia.
Hypoadditive, hyperadditive or additive influences of peripheral–central chemoreceptor interactions?
Our findings clearly demonstrate a synergistic, hyperadditive form of interaction between peripheral and central chemoreceptors in dogs, whereas most previous reports in the literature using isolation of chemoreceptors support a hypoadditive or purely additive interactive effect on ventilatory responsiveness (see Introduction for references). Previous investigations have used an isolated brainstem perfusion (Riedstra, 1963; Kiwull et al.
1972,
1976; Giese et al.
1978) in conjunction with systemic
Figure 4. Individual animal ventilatory responses to central hypercapnia with a background of normal
(filled squares), inhibitory (open squares) and stimulatory (shaded triangles) CB perfusion
Response slopes of minute ventilation, breathing frequency, tidal volume and EMG di to steady-state increases in arterial P
CO
2 with a background of extracorporeal CB perfusion with normal, inhibitory and stimulatory perfusate blood gases are shown for each animal in Table 2.
2010 The Authors. Journal compilation C 2010 The Physiological Society
2464 cyanide injections or hypoxia in anaesthetized cats or rabbits. More recently, Day & Wilson (2007, 2009) used a dual (CB and brainstem) perfusion technique in the decerebrate, vagotomized rat. All of these preparations report that an acidic CNS resulted in a reduced ventilatory response to peripheral chemoreceptor stimulation and, conversely, that a more alkaline central perfusate enhanced peripheral chemosensitivity, i.e. hypoadditive interaction.
The reasons for these qualitative differences between most literature and the present findings have not been systematically explored; however, there are some possible explanations. Firstly, species differences cannot be ruled out, although we know of no specific evidence that would support this explanation. A second possible explanation is that our approach studied the effects of changing CB conditions on the central CO
2 response, whereas most studies showing hypoadditive interaction assessed the response to CB stimulation in a background of altered CNS
G. M. Blain and others J Physiol 588.13
CO
2
/H + (also see “Implications of peripheral–central
. . .
” below). Our studies also included only CB chemoreceptor effects on the central response to hypercapnia, whereas some others included central hypo- and hypercapnia (Heeringa et al.
1979; Day & Wilson, 2007,
2009). A third possibility is the significant reduction in ventilatory reflex chemosensitivity caused by anaesthesia that has been observed in most species studied (Heeringa et al.
1980; Hickey & Severinghaus, 1981; Hwang et al.
1983) or reported in studies using decerebration in conjunction with separate perfusion of the brain and periphery (Day & Wilson, 2009) in the rat. In the rat, the increased respiratory-associated neural activity caused by decerebration (Hayashi & Sinclair, 1991) could potentially contribute to ‘neural saturation’ of respiratory motor output in the face of superimposed chemoreceptor stimulation, as observed in the cat (Eldridge et al.
1981); however, a recent study using decerebration in rats did
Figure 5. Mean ventilatory responses to specific central hypercapnia with a background of normal, inhibitory and stimulatory CB perfusion
Mean values of the minute ventilation ( A ), tidal volume ( B ), breathing frequency ( C ) and mean electrical activity of the costal diaphragm electromyogram ( D ) response slopes to step increases in arterial (and therefore central)
P
CO
2 with a background of extracorporeal carotid body (CB) perfusion with normal ( n = 8), inhibitory ( stimulatory ( n = 4) blood gases. All eight animals are represented in the normal CB central CO
2 n = 6) and response mean line. Given the differing number of animals in each group, the mean percentage change in slope relative to CB normal was calculated using only the data for the animals that were represented in the CB inhibition ( n = 6) or
CB stimulation ( n = 4) groups. See main text for details.
2010 The Authors. Journal compilation C 2010 The Physiological Society
J Physiol 588.13
Peripheral–central chemoreceptor interdependence 2465
Table 2. Individual ventilatory and EMG di
(
(
P
P
CBCO
2
CBCO
2 response slopes to central hypercapnia during CB perfusion with normal
≈
≈
40 mmHg and
40 mmHg; P
P
CBO
2
CBO
2
≈
100 mmHg), inhibitory ( P
CBCO
2
≈
20 mmHg and
≈
40 mmHg) perfusate blood gases and pH
P
CBO
2
>
500 mmHg) and stimulatory
Dog number 1 2 3 4 5 6 7 8 All
Minute ventilation (l min − 1 mmHg − 1
)
CB normal a
R
2
0
0
.
.
52
83
CB inhibition
CB stimulation a
R
2
R a
2
0
0
.
.
07
16
1
.
06
0
.
86
Tidal volume (l mmHg − 1 )
CB normal a
R
2
CB inhibition
CB stimulation a
R
2 a
R
2
0
.
011
0
.
13
0
.
001
0
.
01
0
.
029
0
.
73
0
0
0
0
0
0
.
.
.
.
.
.
047
75
019
27
055
67
Breathing frequency (breaths min − 1
CB normal a
R
2
0
.
66
CB inhibition a
R
2
0
.
62
0
.
11
0
.
04 mmHg − 1 )
0
.
23
−
0
0
0
.
.
.
15
16
21
CB stimulation a
R
2
0
.
85
0
.
81
0
.
51
0
.
68
EMG di
(a.u. mmHg
CB normal
− 1 ) a
R
2
CB inhibition
CB stimulation a
R
2 a
R
2
0
.
061
0
.
95
0
.
016
0
.
28
0
.
080
0
.
99
0
.
48
0
.
32
0
.
06
0
.
02
1
.
10
0
.
75
0
.
048
0
.
55
0
.
023
0
.
47
0
.
120
0
.
95
1
0
0
0
0
0
0
0
1
.
.
.
.
.
.
.
.
.
41
99
44
57
—
—
014
95
005
06
—
—
70
0
.
97
0
.
63
0
.
84
—
—
0
.
075
0
.
98
0
.
018
0
.
37
—
—
1
0
0
0
0
0
0
0
0
0
.
71
0
.
076
0
.
12
0
.
.
.
.
.
.
.
.
.
.
11
71
25
78
—
—
028
68
012
63
—
—
81
—
—
090
0
.
98
0
.
022
0
.
44
—
—
−
−
0
0
0
0
0
0
0
0
0
0
.
99
0
.
91
0
.
013
0
.
.
.
.
—
—
.
.
.
.
—
—
.
—
—
.
5
83
01
02
039
84
006
10
41
043
0
.
51
0
.
016
0
.
70
—
—
0
0
0
0
0
0
0
0
0
0
.
.
.
.
.
.
.
.
.
.
72
83
28
81
—
—
025
66
017
58
—
—
60
0
.
9
0
.
11
0
.
23
—
—
026
0
.
25
0
.
031
0
.
35
—
—
0
0
0
0
0
0
0
0
0
0
.
09
—
0
.
42
0
.
87
0
.
.
.
.
.
.
.
.
.
.
43
65
—
—
96
99
034
62
—
—
053
97
15
—
040
0
.
61
—
—
0
.
088
0
.
98
0
0
0
0
0
0
0
0
0
0
.
.
.
.
.
.
.
.
.
.
37
43
—
—
87
75
021
46
—
—
029
74
19
0
.
26
—
—
0
.
60
0
.
69
039
0
.
17
—
—
0
.
081
0
.
22
0.69
0.70
0.18
0.39
1.00
0.84
0.027
0.64
0.008
0.27
0.041
0.78
0.59
0.59
0.28
0.24
0.59
0.76
0.053
0.63
0.021
0.44
0.092
0.79
The slope coefficient (a) and regression coefficient ( R
2 ) represent linear regression of the data. For other abbreviations, see footnote to Table 1.
not observe saturation, i.e. an alinearity in response, although the response sensitivity was greatly reduced relative to the intact, awake animal (Day & Wilson,
2009). Decerebration might also remove important hypothalamic sites of CO
2
/H + sensitivity, as shown in the cat and the rat (Dillon & Waldrop, 1993; Douglas et al.
2001; Nattie & Li, 2010), and also of other respiratory drives that impinge on CO
2
-sensitive neurons in the retrotrapezoid nucleus (RTN), as shown in the rat (Reddy et al.
2005; Fortuna et al.
2009). Vagotomy has been used in some studies, and it has been shown that vagal denervation can reduce the activity of central RTN CO
2
-sensitive neurons in the rat (Moreira et al.
2007). Hypoadditive effects of an alkaline CSF perfusate (and resultant hypoventilation) on enhancing the transient ventilatory response to systemic cyanide have been reported in the awake goat (Smith et al.
1984); however, these findings were confounded by the potential multiplicative effects of an enhanced background arterial P receptor responsiveness to cyanide.
CO
2 on carotid chemo-
Finally, consistent with our present observations, hyperadditive effects of chemoreceptor interactions on the ventilatory response to hypercapnia have been inferred from the following studies: (1) from the progressive linear responses of phrenic nerve activity to hypercapnia
(applied simultaneously at both the carotid body and central chemoreceptors), which were out of proportion to the increase in carotid sinus nerve activity in cats
(Lahiri & DeLaney, 1975 b ); (2) from the increased slope of the tidal volume response to carotid sinus nerve electrical stimulation resulting from increased [H + ] in the medullary interstitial fluid perfusate in cats (Loeschcke et al.
1963); (3) from cross-circulation experiments in anaesthetized dogs, which showed a synergistic effect of CB hypoxia on the ventilatory response to central hypercapnia
(Adams et al.
1978); and (4) from the reduced ventilatory responses to focal medullary acidosis incurred by bilateral
CBX in awake goats (Hodges et al.
2005).
In summary, clearly there are marked differences between our findings and many, but certainly not all,
2010 The Authors. Journal compilation C 2010 The Physiological Society
2466 previous reports in the literature, which have used approaches involving separate chemoreceptor perfusions to test for chemoreceptor interactions. It is important that more specific comparisons among these disparate models be carried out. We emphasize that our findings of a hyperadditive ventilatory interaction of peripheral and central chemoreceptors are based on studies carried out over a wide range of steady-state carotid body chemoreceptor inhibition and stimulation in an animal model that preserves the essential control system sensitivities and sensory inputs present in the intact, unanaesthetized animal. Based on the foregoing discussion concerning the effects of various types of sensory denervations, CNS lesions and/or anaesthesia on chemoreceptor sensitivities and on key neural pathways, we propose that intact, physiologically responsive preparations are probably required to reveal the true nature of chemoreceptor interaction on cardiorespiratory control and certainly to quantify the magnitude of these effects over ranges of ventilation encountered in normal, physiological conditions.
G. M. Blain and others J Physiol 588.13
response slope (see Fig. 5). Whether a stimulus–response relationship exists for CB hypoxia versus central CO
2 responsiveness remains to be determined.
Finally, we note the substantial variation among our dogs in the magnitude of the synergistic influence of carotid body input on central CO
2 responsiveness
(see Fig. 3 and Table 2). This variability is consistent with well-known interindividual variability of CO
2 responsiveness in both the entire ventilatory control system per se in dogs and humans (Read, 1967; Plum,
1973) and with each set of chemoreceptors when studied separately in the dog (Smith et al.
2006). We would predict that variation in the magnitude of the peripheral–central interactive effect would contribute significantly to the individual variability in overall control system CO
2 responsiveness.
Magnitude and variability of the synergistic interactions between chemoreceptors in the intact animal
We found that marked inhibition of the isolated carotid chemoreceptor via CB hyperoxic hypocapnia reduced the ventilatory response to central CO
2 by 70–100% (see Figs 4 and 5). This effect of CB inhibition is consistent with the reduction in the ventilatory response to central hypercapnia previously reported to occur several days following bilateral carotid body denervation in awake goats and dogs
(Bisgard et al.
1976; Pan et al.
1998; Forster et al.
2000;
Rodman et al.
2001) and in humans (Bellville et al.
1979;
Whipp & Ward, 1992) but exceeds the denervation effects by two- to threefold. We believe the present effects of acute CB inhibition reflect the considerable strength of peripheral–central chemoreceptor synergism inherent in the intact canine respiratory control system. We speculate that the substantially smaller effects on central CO
2 responsiveness resulting from bilateral CB denervation point to the compensatory neural plasticity achieved over time following sensory denervation (Bisgard et al.
1980;
Hoop et al.
1990; Serra et al.
2001, 2002; Forster, 2003; Liu et al.
2003; Hodges et al.
2005). This contrast in findings is also consistent with the substantially greater reduction in eupnoeic air-breathing ventilation found immediately upon acute inhibition of intact carotid chemoreceptors in dogs (Blain et al.
2009) versus that obtained days to months after bilateral CBX.
On the stimulatory side, we observed that a relatively moderate level of carotid body hypoxaemia elicited a twofold average increase in the central CO
2 ventilatory
Implications of peripheral–central chemoreceptor interactions for cardiorespiratory control
Several important consequences of peripheral–central chemoreceptor interaction beyond those shown in the present study remain to be explored in the intact, physiologically responsive preparation. For example, we have only defined the hyperadditive influences of peripheral chemoreceptor inhibition/stimulation on central CO
2 responsiveness, but do not know whether primary central chemoreceptor inhibition/stimulation will exert similar types of synergistic effects on the ventilatory responses to peripheral chemoreceptor stimulation. Studies to date, albeit in reduced preparations, have all reported that a background of altered central [H + ] exerts a hypoadditive interactive effect on the ventilatory response to carotid chemoreceptor stimulation (see Introduction and Discussion above). Also, we need to determine whether physiological means of carotid chemoreceptor stimulation other than hypoxaemia, such as carotid body hypercapnia or asphyxia, increases in [H + ] or [K + ], angiotensin, etc. (Lahiri & DeLaney, 1975 a ; Heinert et al.
1995; Summers et al.
2002; Li & Schultz, 2006) will also elicit hyperadditive effects on central CO
2 sensitivity.
Furthermore, the influence of CNS-specific hypoxia on ventilatory output in unanaesthetized animals appears to be either excitatory or without effect in the absence of carotid chemoreceptors (Davenport et al.
1947; Bouverot et al.
1973; Krasney et al.
1973; Bouverot & Bureau, 1975;
Forster et al.
1976; Bowes et al.
1981; Lahiri et al.
1981;
Smith et al.
1986; Olson et al.
1988; Long et al.
1993), but stimulatory to breathing when the carotid chemoreceptors are intact and maintained in normal conditions (Weizhen et al.
1992; Smith et al.
1993; Curran et al.
2000). These data might be explained by peripheral chemoreceptor afferent influences on central hypoxic ventilatory responsiveness, analogous to what we have currently shown for central
2010 The Authors. Journal compilation C 2010 The Physiological Society
J Physiol 588.13
CO
2
Peripheral–central chemoreceptor interdependence responsiveness. Accordingly, the ventilatory response to acute hypoxic exposure may be dependent in part on peripheral afferent influences on central hypoxic responsiveness, in addition to the established contribution from peripheral chemoreceptors alone.
Our demonstration of hyperadditive influences of carotid body inhibition/stimulation on central CO
2 responsiveness in the dog may have implications for explaining the control of breathing in such varied conditions as normal air-breathing eupnoea, apnoea and states of chronic hyperventilation (see Smith et al.
2010). Firstly, in the dog, for example, during normal spontaneous eupnoea, acute inhibition of carotid chemoreceptors sensory input per se reduces respiratory motor output by more than half its normal air-breathing value
(Blain et al.
2009), an effect which present findings now allow us to attribute to both a removal of tonic sensory input to the respiratory controller and a marked synergistic inhibition of central chemoreceptor sensitivity. Secondly, sleep unmasks a highly sensitive hypocapnia-induced apnoeic threshold in humans (Skatrud & Dempsey,
1983), which we believe, based on the effects of bilateral carotid body denervation in the dog, requires the presence of carotid chemoreceptors (Nakayama et al.
2003). However, neither the isolated carotid chemoreceptor nor the central chemoreceptors, when studied in isolation, respond with sufficient sensitivity to imposed hypocapnia in the carotid body perfusate to cause apnoea in the sleeping dog (Smith et al.
2006, 2007).
Accordingly, additional interactive influences may be required to explain hypocapnia-induced apnoea. Thirdly, time-dependent ventilatory acclimatization to chronic hypoxia and the subsequent sustained hyperventilation following restoration of normoxia is known to be accompanied by both sensitized carotid chemoreceptors in the goat (Nielsen et al.
1988) and an enhanced ‘central’ responsiveness to a given imposed (electrical) stimulation from the carotid chemoreceptors, as demonstrated in the rat (Dwinell & Powell, 1999). The present findings predict that the latter (central) effect of acclimatization is likely to be a consequence of the former (peripheral sensitization) via their interdependent actions. This synergistic influence may also be expected to exert significant influences on cardiorespiratory acclimatization to other clinical conditions with enhanced carotid chemoreceptor sensitization, such as chronic heart failure (Schultz & Li,
2007) and the chronic intermittent hypoxaemia (Ling et al.
2001; Peng & Prabhakar, 2004) accompanying sleep apnoea.
Finally, our findings and those of some others demonstrating peripheral–central chemoreceptor interactions question the common practice of ‘partitioning’ or contrasting ventilatory CO
2 responsiveness between peripheral and central chemoreceptors using such approaches as carotid body denervation in dogs and goats (Pan et al.
1998; Rodman et al.
2001), background hyperoxia in humans (Read, 1967) and/or temporal responses to step increases in F
ICO
2 in intact animals or humans (Fatemian et al.
2003; Smith et al.
2006).
Rather, we suggest that hypercapnic stimulation of the carotid chemoreceptors will lead to a changed central
CO
2 responsiveness, thereby precluding the feasibility of quantifying separate chemoreceptor sensitivities. We caution, however, that thus far we have only demonstrated this hyperadditive interactive effect on the stimulus side with carotid body hypoxia and, as noted above, we now need to determine whether this peripheral–central interactive effect also holds for carotid body hypercapnia.
Sites of peripheral–central chemoreceptor interaction
Potential sites of convergence between carotid body and brainstem chemoreceptors include nucleus tractus solitarii (NTS), RTN and lateral parabrachial nucleus
(LPBN). The RTN is characterized by chemosensitive glutamatergic interneurons, which strongly express
Phox2b (paired-like homeobox 2b gene). These Phox2b neurons are part of an “uninterrupted” chain of neurons in a circuit that includes the CBs and their chemoreceptor afferents, chemosensitive NTS projections to the ventrolateral medulla and RTN central chemoreceptors in the rat (Stornetta et al.
2006; Guyenet,
2008). The CO
2
-sensitive neurons of the RTN in this species comprise a site of convergence for several inputs involved in ventilatory control, including the carotid chemoreceptors (Takakura et al.
2006), vagally mediated pulmonary stretch receptors (Moreira et al.
2007), and input from areas of the hypothalamus associated with the ‘central command’ stimulus of exercise hyperpnoea (Fortuna et al.
2009). The synergistic ventilatory responsiveness to central hypercapnia induced in our study by CB inhibition/stimulation could have occurred at one or more of the above-mentioned chemosensitive sites and/or downstream at medullary sites concerned with generating the respiratory rhythm/ pattern.
Summary
2467
If our findings in the dog can be extrapolated to other species, we would suggest that the conventional view of the chemical control of breathing as a function of separate peripheral and central chemoreceptor sensitivities and their respective stimulus levels in arterial blood and brain may not be justified. Rather, our data in an unanaesthetized canine model show that peripheral chemoreceptor inputs have marked synergistic, hyperadditive influences on central chemoreceptor ventilatory sensitivities to central CO
2
. Thus the magnitude of ventilatory responses and/or adaptations to acute and
2010 The Authors. Journal compilation C 2010 The Physiological Society
2468 chronic chemoreceptor stimuli will probably be a function of the strength of peripheral–central chemoreceptor interactions as well as of individual chemoreceptor stimuli and sensitivities.
References
Adams JM, Attinger FM & Attinger EO (1978). Medullary and carotid chemoreceptor interaction for mild stimuli.
Pflugers
Arch 374 , 39–45.
Adams JM & Severns ML (1982). Interaction of chemoreceptor effects and its dependence on the intensity of stimuli.
J Appl
Physiol 52 , 602–606.
Bellville JW, Whipp BJ, Kaufman RD, Swanson GD, Aqleh KA
& Wiberg DM (1979). Central and peripheral chemoreflex loop gain in normal and carotid body-resected subjects.
J
Appl Physiol 46 , 843–853.
Bisgard GE, Forster HV & Klein JP (1980). Recovery of peripheral chemoreceptor function after denervation in ponies.
J Appl Physiol 49 , 964–970.
Bisgard GE, Forster HV, Orr JA, Buss DD, Rawlings CA &
Rasmussen B (1976). Hypoventilation in ponies after carotid body denervation.
J Appl Physiol 40 , 184–190.
Blain GM, Smith CA, Henderson KS & Dempsey JA (2009).
Contribution of the carotid body chemoreceptors to eupneic ventilation in the intact, unanesthetized dog.
J Appl Physiol
106 , 1564–1573.
Bouverot P & Bureau M (1975). Ventilatory acclimatization and csf acid-base balance in carotid chemodenervated dogs at 3550 m.
Pflugers Arch 361 , 17–23.
Bouverot P, Candas V & Libert JP (1973). Role of the arterial chemoreceptors in ventilatory adaptation to hypoxia of awake dogs and rabbits.
Respir Physiol 17 ,
209–219.
Bowes G, Townsend ER, Kozar LF, Bromley SM & Phillipson
EA (1981). Effect of carotid body denervation on arousal response to hypoxia in sleeping dogs.
J Appl Physiol 51 ,
40–45.
Clement ID, Bascom DA & Robbins PA (1992). An assessment of central-peripheral ventilatory chemoreflex interaction in humans.
Respir Physiol 88 , 87–100.
Clement ID, Pandit JJ, Bascom DA, Dorrington KL, O’Connor
DF & Robbins PA (1995). An assessment of central–peripheral ventilatory chemoreflex interaction using acid and bicarbonate infusions in humans.
J Physiol 485 ,
561–570.
Comroe JH Jr & Schmidt CF (1938). The part played by reflexes from the carotid body in the chemical regulation of respiration in the dog.
Am J Physiol 121 , 75–97.
Curran AK, Rodman JR, Eastwood PR, Henderson KS,
Dempsey JA & Smith CA (2000). Ventilatory responses to specific CNS hypoxia in sleeping dogs.
J Appl Physiol 88 ,
1840–1852.
Dahan A, Van Den Elsen MJ, Berkenbosch A, DeGoede J,
Olievier IC, van Kleef JW & Bovill JG (1994). Effects of subanesthetic halothane on the ventilatory responses to hypercapnia and acute hypoxia in healthy volunteers.
Anesthesiology 80 , 727–738.
G. M. Blain and others J Physiol 588.13
Daristotle L & Bisgard GE (1989). Central-peripheral chemoreceptor ventilatory interaction in awake goats.
Respir
Physiol 76 , 383–391.
Davenport HW, Brewer G, Chambers AH & Goldschmidt S
(1947). The respiratory responses to anoxemia of unanesthetized dogs with chronically denervated aortic and carotid chemoreceptors and their causes.
Am J Physiol 148 ,
406–416.
Day TA & Wilson RJ (2007). Brainstem P
CO
2 modulates phrenic responses to specific carotid body hypoxia in an in situ dual perfused rat preparation.
J Physiol 578 , 843–857.
Day TA & Wilson RJA (2009). A negative interaction between brainstem and peripheral respiratory chemoreceptors modulates peripheral chemoreflex magnitude.
J Physiol 587 ,
883–896.
Dempsey JA & Forster HV (1982). Mediation of ventilatory adaptations.
Physiol Rev 62 , 262–346.
Dillon GH & Waldrop TG (1993). Responses of feline caudal hypothalamic cardiorespiratory neurons to hypoxia and hypercapnia.
Exp Brain Res 96 , 260–272.
Douglas RM, Trouth CO, James SD, Sexcius LM, Kc P,
Dehkordi O, Valladares ER & McKenzie JC (2001).
Decreased CSF pH at ventral brain stem induces widespread c-Fos immunoreactivity in rat brain neurons.
J Appl Physiol
90 , 475–485.
Drummond GB (2009). Reporting ethical matters in The
Journal of Physiology : standards and advice.
J Physiol 587 ,
713–719.
Dutton RE, Hodson WA, Davies DG & Chernick V (1967).
Ventilatory adaptation to a step change in PCO
2 at the carotid bodies.
J Appl Physiol 23 , 195–202.
Dwinell MR & Powell FL (1999). Chronic hypoxia enhances the phrenic nerve response to arterial chemoreceptor stimulation in anesthetized rats.
J Appl Physiol 87 , 817–823.
Eldridge FL & Gill-Kumar P (1980). Central neural respiratory drive and afterdischarge.
Respir Physiol 40 , 49–63.
Eldridge FL, Gill-Kumar P & Millhorn DE (1981).
Input–output relationships of central neural circuits involved in respiration in cats.
J Physiol 311 , 81–95.
Fatemian M, Nieuwenhuijs DJ, Teppema LJ, Meinesz S, Van
Der Mey AG, Dahan A & Robbins PA (2003). The respiratory response to carbon dioxide in humans with unilateral and bilateral resections of the carotid bodies.
J Physiol 549 ,
965–973.
Fencl V, Miller TB & Pappenheimer JR (1966). Studies on the respiratory response to disturbances of acid-base balance, with deductions concerning the ionic composition of cerebral interstitial fluid.
Am J Physiol 210 , 459–472.
Forster HV (2003). Plasticity in the control of breathing following sensory denervation.
J Appl Physiol 94 ,
784–794.
Forster HV, Bisgard GE, Rasmussen B, Orr JA, Buss DD &
Manohar M (1976). Ventilatory control in peripheral chemoreceptor-denervated ponies during chronic hypoxemia.
J Appl Physiol 41 , 878–885.
Forster HV, Pan LG, Lowry TF, Serra A, Wenninger J &
Martino P (2000). Important role of carotid chemoreceptor afferents in control of breathing of adult and neonatal mammals.
Respir Physiol 119 , 199–208.
2010 The Authors. Journal compilation C 2010 The Physiological Society
J Physiol 588.13
Fortuna MG, Stornetta RL, West GH & Guyenet PG (2009).
Activation of the retrotrapezoid nucleus by posterior hypothalamic stimulation.
J Physiol 587 , 5121–5138.
Gesell R & Hamilton MA (1941). Reflexogenic components of breathing.
Am J Physiol 133 , 694–719.
Gesell R, Lapides J & Levin M (1940). The interaction of central and peripheral chemical control of breathing.
Am J Physiol
130 , 155–170.
Giese K, Berndt J & Berger W (1978). Interaction of central and peripheral respiratory drives in cats. II. Peripheral and central interaction of hypoxia and hypercapnia.
Pflugers Arch
374 , 211–217.
Guyenet PG (2008). The 2008 Carl Ludwig Lecture: retrotrapezoid nucleus, CO
2 homeostasis, and breathing automaticity.
J Appl Physiol 105 , 404–416.
Guyenet PG, Bayliss DA, Mulkey DK, Stornetta RL,
Moreira TS & Takakura AT (2008 a ). The retrotrapezoid nucleus and central chemoreception.
Adv Exp Med Biol 605 ,
327–332.
Guyenet PG, Stornetta RL & Bayliss DA (2008 b ).
Retrotrapezoid nucleus and central chemoreception.
J
Physiol 586 , 2043–2048.
Haldane JS & Priestly JG (1905). The regulation of the lung-ventilation.
J Physiol 32 , 225–266.
Hayashi F & Sinclair JD (1991). Respiratory patterns in anesthetised rats before and after anemic decerebration.
Respir Physiol 84 , 61–76.
Heeringa J, Berkenbosch A, de Goede J & Olievier CN (1979).
Relative contribution of central and peripheral chemoreceptors to the ventilatory response to CO
2 during hyperoxia.
Respir Physiol 37 , 365–379.
Heeringa J, de Goede J, Berkenbosch A & Olievier CN (1980).
Influence of the depth of anaesthesia on the peripheral and central ventilatory CO
Physiol 41 , 333–347.
2 sensitivity during hyperoxia.
Respir
Heinert G, Paterson DJ, Bisgard GE, Xia N, Painter R & Nye
PCG (1995). The excitation of carotid body chemoreceptors of the cat by potassium and noradrenaline.
Adv Exp Med Biol
393 , 323–330.
Heymans C, Bouckaert J-J & Dautrebande L (1930). Sinus carotidien et reflexes respiratoires. II. Influences respiratoires reflexes de l’acidose, de l’alcalose, de l’anhydride carbonique, de l’ion hydrogene et de l’anoxemie. Sinus carotidien et echanges respiratoires dans les poumons et au dela des poumons.
Arch Intern Pharmacodyn 39 , 400–450.
Hickey RF & Severinghaus JW (1981). Regulation of breathing: drug effects. In Regulation of Breathing Part II , ed. Hornbein
TF, pp. 1251–1312. Marcel Dekker, New York, Basel.
Hodges MR, Opansky C, Qian B, Davis S, Bonis JM, Krause K,
Pan LG & Forster HV (2005). Carotid body denervation alters ventilatory responses to ibotenic acid injections or focal acidosis in the medullary raphe.
J Appl Physiol 98 ,
1234–1242.
Hoop B, Masjedi M, Shih VE & Kazemi H (1990). Brain glutamate metabolism during hypoxia and peripheral chemodenervation.
J Appl Physiol 69 , 147–154.
Hwang JC, St John WM & Bartlett D Jr (1983).
Respiratory-related hypoglossal nerve activity: influence of anesthetics.
J Appl Physiol 55 , 785–792.
Peripheral–central chemoreceptor interdependence 2469
Kiwull P, Wiemer W & Sch¨one H (1972). The role of the carotid chemoreceptors in the CO
2
-hyperpnea under hyperoxia.
Pflugers Arch 336 , 171–186.
Kiwull PH, Kiwull-Schone S & Klatt W (1976). Interaction of central and peripheral respiratory drives: differentiation between the role of stimuli and afferents. In Acid-Base
Homeostasis of the Brain Extracellular Fluid and Respiratory
Control System , ed. Loeschcke HH, pp. 146–156. Thieme,
Stuttgart.
Krasney JA, Magno MG, Levitzky MG, Koehler RC & Davies
DG (1973). Cardiovascular responses to arterial hypoxia in awake sinoaortic-denervated dogs.
J Appl Physiol 35 ,
733–738.
Lahiri S & DeLaney RG (1975 a ). Stimulus interaction in the responses of carotid body chemoreceptor single afferent fibres.
Respir Physiol 24 , 249–266.
Lahiri S & DeLaney RG (1975 b ). Relationship between carotid chemoreceptor activity and ventilation in the cat.
Respir
Physiol 24 , 267–286.
Lahiri S, Edelman NH, Cherniack NS & Fishman AP (1981).
Role of carotid chemoreflex in respiratory acclimatization to hypoxemia in goat and sheep.
Respir Physiol 46 , 367–382.
Li YL & Schultz HD (2006). Enhanced sensitivity of Kv channels to hypoxia in the rabbit carotid body in heart failure: role of angiotensin II.
J Physiol 575 , 215–227.
Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB Jr & Mitchell
GS (2001). Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing.
J Neurosci 21 , 5381–5388.
Liu Q, Kim J, Cinotte J, Homolka P & Wong-Riley MT (2003).
Carotid body denervation effect on cytochrome oxidase activity in pre-B¨otzinger complex of developing rats.
J Appl
Physiol 94 , 1115–1121.
Loeschcke HH, Mitchell RA, Katsaros B, Perkins JF Jr & Konig
A (1963). Interaction of intracranial chemosensitivity with peripheral afferents to the respiratory centres.
Ann N Y Acad
Sci 109 , 651–660.
Long WQ, Giesbrecht GG & Anthonisen NR (1993).
Ventilatory response to moderate hypoxia in awake chemodenervated cats.
J Appl Physiol 74 , 805–810.
Miller JD, Hemauer SJ, Smith CA, Stickland MK & Dempsey JA
(2006). Expiratory threshold loading impairs cardiovascular function in health and chronic heart failure during submaximal exercise.
J Appl Physiol 101 , 213–227.
Mitchell RA (1965). The regulation of respiration in metabolic acidosis and alkalosis. In Cerbrospinal Fluid and the
Regulation of Respiration , ed. Brooks CM, Kao FF & Lloyd
BB, pp. 109–131. Blackwell, Oxford.
Mitchell RA, Loeschcke HH, Severinghaus JW, Richardson BW
& Massion WH (1963). Regions of respiratory chemosensitivity on the surface of the medulla.
Ann N Y
Acad Sci 109 , 661–681.
Moreira TS, Takakura AC, Colombari E, West GH & Guyenet
PG (2007). Inhibitory input from slowly adapting lung stretch receptors to retrotrapezoid nucleus chemoreceptors.
J
Physiol 580 , 285–300.
Musch TI, Pelligrino A & Dempsey JA (1980). Effects of prolonged N
2
O and barbiturate anesthesia on brain metabolism and pH in the dog.
Respir Physiol 39 , 121–131.
2010 The Authors. Journal compilation C 2010 The Physiological Society
2470 G. M. Blain and others
Nakayama H, Smith CA, Rodman JR, Skatrud JB & Dempsey
JA (2003). Carotid body denervation eliminates apnea in response to transient hypocapnia.
J Appl Physiol 94 ,
155–164.
Nattie EE & Li A (2009). Central chemoreception is a complex system function that involves multiple brainstem sites.
J Appl
Physiol 106 , 1464–1466.
Nattie E & Li A (2010). Central chemoreception in wakefulness and sleep: evidence for a distributed network and a role for orexin.
J Appl Physiol 108 , 1417–1424,
DOI: 10.1152/japplphysiol.01261.02009.
Nielsen AM, Bisgard GE & Vidruk EH (1988). Carotid chemoreceptor activity during acute and sustained hypoxia in goats.
J Appl Physiol 65 , 1796–1802.
Olson EB Jr, Vidruk EH & Dempsey JA (1988). Carotid body excision significantly changes ventilatory control in awake rats.
J Appl Physiol 64 , 666–671.
Pan LG, Forster HV, Martino P, Strecker PJ, Beales J, Serra A,
Lowry TF, Forster MM & Forster AL (1998). Important role of carotid afferents in control of breathing.
J Appl Physiol 85 ,
1299–1306.
Peng YJ & Prabhakar NR (2004). Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity.
J Appl Physiol 96 , 1236–1242.
Plum F (1973). Reproducibility of the rebreathing carbon dioxide response test using an improved method.
Am Rev
Respir Dis 107 , 864–867.
Read DJC (1967). A clinical method for assessing the ventilatory response to carbon dioxide.
Australas Ann Med
16 , 20–32.
Reddy MK, Patel KP & Schultz HD (2005). Differential role of the paraventricular nucleus of the hypothalamus in modulating the sympathoexcitatory component of peripheral and central chemoreflexes.
Am J Physiol Regul
Integr Comp Physiol 289 , R789–R797.
Riedstra JW (1963). Influence of central and peripheral PCO
2
(pH) on the ventilatory response to hypoxic chemoreceptor stimulation.
Acta Physiol Pharmacol Neerl 12 , 407–452.
Robbins PA (1988). Evidence for interaction between the contributions to ventilation from the central and peripheral chemoreceptors in man.
J Physiol 401 , 503–518.
Rodman JR, Curran AK, Henderson KS, Dempsey JA & Smith
CA (2001). Carotid body denervation in dogs: eupnea and the ventilatory response to hyperoxic hypercapnia.
J Appl
Physiol 91 , 328–335.
St Croix CM, Cunningham DA & Paterson DH (1996). Nature of the interaction between central and peripheral chemoreceptor drives in human subjects.
Can J Physiol
Pharmacol 74 , 640–646.
Saupe KW, Smith CA, Henderson KS & Dempsey JA (1995).
Effects of raising carotid sinus pressure on upper airway resistance and EEG frequency in sleeping dogs.
J Appl Physiol
78 , 1699–1709.
Schlaefke ME, See WR, Herker-See A & Loeschcke HH (1979).
Respiratory response to hypoxia and hypercapnia after elimination of central chemosensitivity.
Pflugers Arch 381 ,
241–248.
Schultz HD & Li YL (2007). Carotid body function in heart failure.
Respir Physiol Neurobiol 157 , 171–185.
J Physiol 588.13
Serra A, Brozoski D, Hedin N, Franciosi R & Forster HV
(2001). Mortality after carotid body denervation in rats.
J
Appl Physiol 91 , 1298–1306.
Serra A, Brozoski D, Hodges M, Roethle S, Franciosi R &
Forster HV (2002). Effects of carotid and aortic chemoreceptor denervation in newborn piglets.
J Appl
Physiol 92 , 893–900.
Skatrud JB & Dempsey JA (1983). Interaction of sleep state and chemical stimuli in sustaining rhythmic ventilation.
J Appl
Physiol 55 , 813–822.
Smith CA, Bisgard GE, Nielsen AM, Daristotle L, Kressin NA,
Forster HV & Dempsey JA (1986). Carotid bodies are required for ventilatory acclimatization to chronic hypoxia.
J
Appl Physiol 60 , 1003–1010.
Smith CA, Chenuel BJ, Henderson KS & Dempsey JA (2007).
The apneic threshold during non-REM sleep in dogs: sensitivity of carotid body vs. central chemoreceptors.
J Appl
Physiol 103 , 578–586.
Smith CA, Engwall MJ, Dempsey JA & Bisgard GE (1993).
Effects of specific carotid body and brain hypoxia on respiratory muscle control in the awake goat.
J Physiol 460 ,
623–640.
Smith CA, Forster HV, Blain GM & Dempsey JA (2010). An interdependent model of central/peripheral chemoreception:
Evidence and implications for ventilatory control.
Respir
Physiol Neurobiol , DOI: 10.1016/j.resp.2010.02.015.
Smith CA, Jameson LC, Mitchell GS, Musch TI & Dempsey JA
(1984). Central-peripheral chemoreceptor interaction in awake cerebrospinal fluid-perfused goats.
J Appl Physiol 56 ,
1541–1549.
Smith CA, Rodman JR, Chenuel BJ, Henderson KS & Dempsey
JA (2006). Response time and sensitivity of the ventilatory response to CO
2 in unanesthetized intact dogs: central vs.
peripheral chemoreceptors.
J Appl Physiol 100 , 13–19.
Smith CA, Saupe KW, Henderson KS & Dempsey JA (1995).
Ventilatory effects of specific carotid body hypocapnia in dogs during wakefulness and sleep.
J Appl Physiol 79 ,
689–699.
Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA,
West GH, Brunet JF, Mulkey DK, Bayliss DA & Guyenet PG
(2006). Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat.
J Neurosci 26 ,
10305–10314.
Summers BA, Overholt JL & Prabhakar NR (2002). CO
2 pH independently modulate L-type Ca 2 + and current in rabbit carotid body glomus cells.
J Neurophysiol 88 , 604–612.
Takakura AC, Moreira TS, Colombari E, West GH, Stornetta
RL & Guyenet PG (2006). Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO
2
-sensitive neurons in rats.
J Physiol 572 , 503–523.
van Beek JH, Berkenbosch A, de Goede J & Olievier CN (1983).
Influence of peripheral O
2 response to CO
2 tension on the ventilatory in cats.
Respir Physiol 51 , 379–390.
Weizhen N, Engwall MJ, Daristotle L, Pizarro J & Bisgard GE
(1992). Ventilatory effects of prolonged systemic (CNS) hypoxia in awake goats.
Respir Physiol 87 , 37–48.
Whipp BJ & Ward SA (1992). Physiologic changes following bilateral carotid-body resection in patients with chronic obstructive pulmonary disease.
Chest 101 , 656–661.
2010 The Authors. Journal compilation C 2010 The Physiological Society
J Physiol 588.13
Peripheral–central chemoreceptor interdependence
Zhang W, Carreno FR, Cunningham JT & Mifflin SW (2009).
Chronic sustained hypoxia enhances both evoked EPSCs and norepinephrine inhibition of glutamatergic afferent inputs in the nucleus of the solitary tract.
J Neurosci 29 ,
3093–3102.
2471
Sciences, School of Medicine and Public Health, University of
Wisconsin-Madison. All authors were involved in study design, collection, analysis and interpretation of the data and writing the paper. All authors approved the final submitted version.
Author contributions
All experiments were performed at The John Rankin Laboratory of Pulmonary Medicine, Department of Population Health
Acknowledgements
This study was supported by grants from the National Heart,
Lung, and Blood Institute of the National Institutes of Health and the American Heart Association.
2010 The Authors. Journal compilation C 2010 The Physiological Society