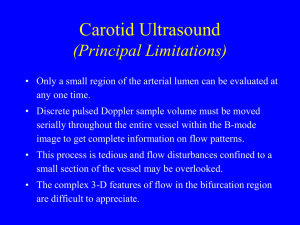
Why do we have both peripheral and central chemoreceptors?
advertisement

J Appl Physiol 100: 9 –10, 2006; doi:10.1152/japplphysiol.01097.2005. Invited Editorial Why do we have both peripheral and central chemoreceptors? The peripheral chemoreceptors, the carotid (and aortic) bodies, detect arterial hypoxemia and stimulate breathing. At normal arterial PO2 (PaO2) values, they provide a tonic excitatory input to the brain stem (6), and with hypoxia they respond dramatically as PaO2 falls below 70 Torr. Thus, for oxygen, they provide an emergency detection system not a breath-by-breath measure of gas-exchange success. Both the carotid bodies and the central chemoreceptors detect changes in CO2/pH and affect breathing. What are their respective roles and importance? These are old questions (see Refs. 3, 5, and 7 for historical perspective), but the paper by Smith et al. (9) in this issue of the Journal of Applied Physiology provides unique data obtained from conscious dogs that support clear answers to them. The dogs have one carotid body denervated (the main peripheral chemoreceptor in the dog) and the other perfused with blood under the control of the investigators. This maintains the tonic input from the carotid body and avoids any time-related changes that occur in animals after denervation (6). They induce a near-square-wave increase in end-tidal PCO2 of 10-12 Torr at 1) both the carotid body and the central chemoreceptors (“CB ⫹ Central”) or 2) only the central chemoreceptors (“Central Only”) with the carotid body simultaneously perfused with blood of normal PO2, PCO2, and pH. There are two protocols: 1) several 1- to 2-min exposures, and 2) 5-min steady-state exposures. The results are remarkable and clear. With Central Only stimulation, the response (a value of ventilation ⬎3 SDs above baseline) was delayed by 11.2 s in the rapid transient protocol, but in the steady-state experiments the response was 63% of that with CB ⫹ CCR stimulation. The carotid bodies provide the rapid response, but the central chemoreceptors provide most of the steady-state response. The greater role of the central chemoreceptors in the steadystate response is in good agreement with a large number of previous studies that used either acute carotid body denervation or separate perfusion of the two sites in anesthetized animals. The greater role of the carotid bodies in the rapid response complements their earlier results linking transient hypocapnia at the carotid body to hypoventilation and apnea in awake and sleeping dogs (3). Studies in conscious humans with intact or surgically resected carotid bodies provide strong support for this rapid response function of the carotid body. Fatemian et al. (4) used a multifrequency binary sequence to force brief CO2 pulses and determine both a fast and slow time constant for the CO2 response. The fast time constant was absent in the patients with carotid body resection. That these two very different approaches, both without the depressant effect of anesthesia, produce the same qualitative result is powerful evidence that the carotid body is a rapid arterial CO2 detection system that can monitor the effectiveness of alveolar ventilation. However, Smith et al. (9) qualify this result, unnecessarily in my view. They refer to experiments performed in deeply anesthetized animals with carotid body denervation, surgical exposure of the ventral medullary surface (the putative central chemoreceptor site), and measurement of surface pH [see Refs. 1, 9, 18, 22, 33 in Smith et al. (9)]. Here, large step changes in PCO2 increase fictive breathing (phrenic nerve activity) and http://www. jap.org decrease ventral medullary surface “extracellular fluid” pH relatively quickly. However, the surgery is drastic, and the condition of the medullary surface and its blood flow is questionable [see discussion by Cragg et al. (2)]. Furthermore, the surface pH response time to a step change in end-tidal CO2 is similar in animals with carotid body denervation [see Refs. 1 and 33 in Smith et al. (9)]. These experiments show that central chemoreceptors detect pH in interstitial fluid but not in cerebrospinal fluid. However, in my view, it is unreasonable to compare response dynamics obtained from experiments of this nature with those obtained in conscious dogs and humans. What do central chemoreceptors sense? Some have proposed that they detect arterial CO2. For example, the proximity of serotonergic neurons (one putative central chemoreceptor) to large arteries suggests this view (1). However, the difference in time for a step change in end-tidal PCO2 to reach the carotid artery vs. the arteries that supply putative central chemoreceptor neurons should be but a few seconds. If central chemoreceptors detect arterial CO2, then why the large difference in response times (4, 9)? Perhaps the dynamics of the neural response to the detected signal differ. Alternatively, the central chemoreceptors detect a signal other than arterial CO2. There is strong support for this idea. Pappenheimer and colleagues (5) used ventriculocisternal perfusion and sustained systemic acidbase changes in chronic unanesthetized goats to correlate steady-state ventilatory responses with pH values in different compartments. They concluded that central chemoreceptors detect brain interstitial fluid pH. In this case, central chemoreceptors are sensitive to changes in arterial PCO2, cerebral blood flow, and cerebral metabolism. Disturbances in any of these three entities would change interstitial fluid pH and, thereby, ventilation. That primary changes in cerebral blood flow can affect breathing, via central chemoreceptors, independent of any change in PaCO2 supports this view (see Ref. 10). And, in conscious animals, the cerebral blood flow response to CO2 will modulate the response dynamics. Thus the combined recent work of Dempsey, Smith, Skatrud, and colleagues and older work of Pappenheimer and colleagues, all in conscious animals, results in a clear picture of chemoreception. The carotid bodies detect arterial CO2 (and pH) and monitor alveolar ventilation while the central chemoreceptors detect interstitial fluid pH and monitor the balance of arterial CO2, cerebral blood flow, and cerebral metabolism. This balance also provides an indirect index of the balance of cerebral oxygen delivery and utilization; that is, central chemoreceptors can act as indirect brain tissue oxygen sensors. This view gives serious purpose to central chemoreceptors and may explain why they are located at many sites (8). They are not a mere back-up for the carotid body, which seems perfectly adequate as a detector of arterial CO2 in physiological conditions, are especially suited for rapid responses (3, 4, 9), and provide a needed tonic drive under normal CO2/pH conditions (6). REFERENCES 1. Bradley SR, Pieribone VA, Wang W, Severson CA, Jacobs RA, and Richerson GB. Chemosensitive serotonergic neurons are closely associated with large medullary arteries. Nat Neurosci 5: 401– 402, 2002. 8750-7587/06 $8.00 Copyright © 2006 the American Physiological Society 9 Invited Editorial 10 PERIPHERAL AND CENTRAL CHEMORECEPTION 2. Cragg P, Patterson L, and Purves MJ. The pH of brain extracellular fluid in the cat. J Physiol 272: 137–166, 1977. 3. Dempsey JA. Crossing the apnoeic threshold: causes and consequences. (The Julius H Comroe Lecture). Exp Physiol 90: 13–24, 2004. 4. Fatemian M, Nieuwenhuijs DJF, Teppema LJ, Meinesz S, van der Mey AGJ, Dahan A, and Robbins PA. The respiratory response to carbon dioxide in humans with unilateral and bilateral resections of the carotid bodies. J Physiol 549: 965–973, 2003. 5. Fencl V, Miller TB, and Pappenheimer JR. Studies on the respiratory response to disturbances of acid-base balance, with deductions concerning the ionic composition of cerebral interstitial fluid. Am J Physiol 210: 459 – 472, 1966. 6. Forster HV, Pan LG, Lowry TF, Serra A, Wenninger J, and Martino P. Important role of carotid chemoreceptor afferents in control of breathing in adult and neonatal mammals. Respir Physiol 119: 199 –208, 2000. 7. Loeschcke HH. Central chemosensitivity and the reaction theory. J Physiol 332: 1–24, 1982. J Appl Physiol • VOL 8. Nattie EE. Multiple sites for central chemoreception: their roles in response sensitivity and in sleep and wakefulness (Review). Respir Physiol 122: 223–235, 2000. 9. Smith CA, Rodman JR, Chenuel BJA, Henderson KS, and Dempsey JA. Response time and sensitivity of the ventilatory response to CO2 in unanesthetized intact dogs: central vs. peripheral chemoreceptors. J Appl Physiol 100: 13–19, 2006. 10. Xie A, Skatrud JB, Khayat R, Dempsey JA, Morgan B, and Russell D. Cerebrovascular response to carbon dioxide in patients with congestive heart failure. Am J Respir Crit Care Med 172: 371–378, 2005. 100 • JANUARY 2006 • Eugene Nattie Department of Physiology Dartmouth Medical School Lebanon, New Hampshire e-mail: eugene.nattie@dartmouth.edu www.jap.org