Genetics & Genomics Practice Problems: Restriction Mapping, RFLPs
advertisement

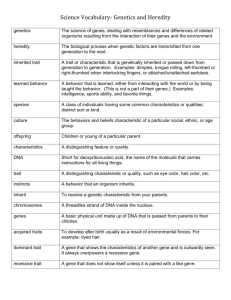
BS 50—Genetics and Genomics Week of Nov 29 Additional Practice Problems for Section Problem 1. A linear piece of DNA is digested with restriction enzymes EcoRI and HinDIII, and the products are separated on an agarose gel. The results for the single and double digests are shown below (size of fragments in kilobases, kb). EcoRI 7.5 2 1 HinDIII 5.5 5 EcoRI+HinDIII 4.5 3 2 1 a. What is the original length of the undigested DNA molecule? b. How many EcoRI sites are in the molecule? c. Draw a restriction map of the DNA molecule, indicating the site of each restriction enzyme site. The line below has a long line at each kb and a short line at every 0.5 kb. Use the line below to draw the linear DNA molecule and make all RE site marks on the scale marks provided. Start the map at the left end of the line (“Start”). Please note that you may not use the entire line for your map, so you should also indicate where your DNA molecule ends on the map (“End”). Start Problem 2 (20 pts). You are studying a rare single gene hereditary disease (SIK) in a mouse model. You have a plasmid in the lab that carries DNA from the region near the disease gene sik. To map the DNA clone relative to the sik gene, a restriction fragment length polymorphism is identified. Your plasmid DNA clone hybridizes to a 10kb fragment in purebreeding strain A. It also hybridizes to two fragments, 7kb and 3kb, in purebreeding strain B. An affected strain A female is mated with an unaffected strain B male, and their children are then mated with each other. The pedigree and Southern blot are shown below: 1 I II 2 3 4 5 6 7 8 9 III 10kb 7kb 3kb A) What is the mode of inheritance for the SIK phenotype? B) What are the genotypes for the following individuals? Include both the sik and the RFLP genotypes in your answer: II-3: III-9: C) What is the most likely explanation for the RFLP pattern seen in male III-9? Problem 3 (18 pts) You are studying a human trait and have discovered a restriction fragment length polymorphism (RFLP) associated with the disease. Use the pedigree from the following family along with the Southern blot analyzing inheritance of the RFLP to answer the following questions. A B A B C D E F G 11 11 kb 7 6 7 kb 6 kb 3 1 3 kb 1 kb a) Just by looking at the pedigree, (and ignoring the DNA data,) can you determine whether the trait is dominant or recessive? Sex-linked or autosomal? Explain your reasoning. b) Using the DNA data as well, is this trait dominant or recessive? Sex-linked or autosomal? Explain. c) Individual F marries a phenotypically “normal” male. Their child is found to have only a 3 kb and 1 kb band when analyzed with the same probe used above. Assuming the RFLP is actually 1 cM from the gene for this trait, what is the probability that their child will be male and have this trait? Problem 4. A RFLP is discovered that is linked to the gene for Duchenne’s muscular dystrophy (DMD). DMD is an X-linked, recessive trait. The RFLP is 2 map units from the gene for DMD. Consider the following pedigree and Southern blot using a probe that hybridizes to the RFLP. 1 2 3 4 5 6 7 8 9 10kb 8kb 2kb 1 2 3 4 5 6 7 8 9 Individuals 8 and 9 have both married men who do NOT have muscular dystrophy, and both women are pregnant. Remember, DMD is an X-linked trait. a. If you did not know the RFLP information, what is the predicted probability that individual 8’s child might have muscular dystrophy? Show your reasoning. b. Knowing the RFLP information, what is the probability that individual 9’s child will have muscular dystrophy? Show your reasoning. An amniocentesis is performed and it is determined that 9’s child in utero has only a 10 kb band that hybridizes to the same probe used above. How does this change your answer to part B? bp 20 0 yfg TetR yfg = gene orientation for yfg HinDIII EcoRI BamHI 800b p EcoRI BamHI HinDIII EcoRI Problem 5. lacZ AmpR = Inducible Promoter You decide to clone your favorite gene (yfg) into a 6000bp target plasmid that contains a special inducible promoter. Therefore, in order to achieve high expression of yfg, you must clone yfg in the same orientation as the promoter in the target vector (arrowheads must point in the same direction). You have already mapped the plasmid for three sticky-end restriction enzymes as shown above (assume a negligible distance between the sites in the target vector multiple cloning site within lacZ). You also note that yfg has a HinDIII site that cuts yfg into a 200bp fragment and an 800bp fragment in your TetR vector when double digested with HinDIII and EcoRI. a. What enzyme(s) do you use to cut the target vector for cloning yfg into it? Briefly justify your choice. b. Using your strategy, will yfg insert into the target vector in the same orientation as the promoter always, sometimes, or never? Briefly justify your answer. c. Which single restriction enzyme can be used to conclusively confirm that yfg is cloned in the correct orientation in the target vector? Briefly justify by explaining what results you expect to see if yfg is in the correct and in the incorrect orientation. Question 6. Look at the gel below, which was generated by the dideoxy sequencing method. a) Deduce the nucleotide sequence of the DNA nucleotide chain synthesized from the primer. Label the 5’and 3’ ends. b) Deduce the nucleotide sequence of the DNA nucleotide chain used as the template strand. Label the 5’ and 3’ ends. c) How many possible reading frames are there? d) How many of the reading frames are “open,” based on the sequence available?