Spectroscopy
advertisement

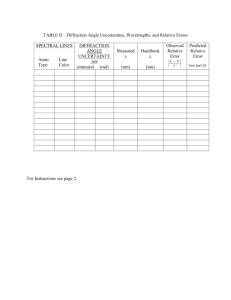
Astro 452 Fall 2004 Spectroscopy 1. Introduction The purpose of the spectroscopy experiment is to introduce you to the operation of a simple spectrometer and to the measurement of the wavelengths of spectral lines. To this end you are asked to carry out the exercise of measuring the refractive index of glass at a number of different wavelengths. This goal is accomplished in a series of experimental steps, each of which depends on the outcome of the previous step. The grand plan is as follows: (a) Use the spectrometer with a transmission grating to measure the deflection angles of the first few spectral lines of the Balmer series of Hydrogen. Since the wavelengths of these lines can be calculated from theory, their deflection angles can be used to “calibrate” the grating. (b) With the grating “calibrated”, use the spectrometer to measure the wavelengths of some of the bright spectral lines in the spectrum of Helium. (c) The combined set of the lines of Hydrogen and Helium, whose wavelengths are known or have been measured are used as monochromatic light sources to measure the refractive index of glass. The grating is replaced by a glass prism as the dispersive device in the spectrometer and the deflection angle of light in the different spectral lines is measured. Since the deflection angle depends on the refractive index at that wavelength, the refractive index can be determined. The next setion below is a guide to calculating the wavelengths of the spectral lines of Hydrogen. This calculation must be done before coming to the lab. The section following that describes, as the spectrometer, its alignment procedure, and its operation, as well as the theoretical principles of dispersion by a grating and a prism. Later sections give the details of the experimental exercises. You have two weeks to carry out this experiment. You should submit a single lab report with all of the results at the end of the two weeks. 2. The Wavelengths of the Spectral Lines of Hydrogen Hydrogen is the simplest atom and it is reasonably well understood. Its atomic energy levels can be calculated from theory, hence so can the wavelengths of its spectral lines. The formula that gives the wavelengths of the spectral lines is ! 1 1 1 − 2 , =R λ n2f ni where ni and nf are the initial and final energy levels of an electronic transition and R = 1.09709 × 107 m−1 is known as the Rydberg constant. The Balmer series of lines have wavelengths that fall in the visible part of the spectrum. This series corresponds to transitions to nf = 2. Calculate the wavelengths of the first four lines in the Balmer series: –1– Astro 452 Spectroscopy Fall 2004 Figure 1: – A schematic of the spectrometer with the main parts labelled. The thin dahed line marks the light path for dispersed light, with the deflection angle labelled as θ . –2– Astro 452 Spectroscopy Fall 2004 Hα, Hβ, Hγ, and Hδ (ni = 3, 4, 5, 6). Express the wavelengths in Å. These are the lines that you should expect to see in the first part of the experiment. 3. The Spectrometer 3.1 Description A schematic of the spectrometer that you will use in the lab is shown in Figure 1. The main elements are: The Collimator: It delivers a parallel beam of light from the source to the disperser (e.g., a grating). It has a narrow slit at one end and a lens at the other end. The focusing knob is used to adjust the length of the tube so that the slit is at the focus of the collimator’s objective lens. The Table: It is a flat surface on which the disperser is mounted and secured with a holder. It also has a fixed angular vernier scale which is used to measure the angle of the telescope as it swings around to observe the dispersed light. There are three screws/knobs associated with the table. One is high up, just below the tabletop and it is used to secure the table onto the base. By loosening this screw you can move the table up or down as well as rotate it. The other two screws/knobs are close to the bottom of the base and they are used to move and secure the internal table assembly. Once you adjust the the orientation and level of the table tighten the screws and do not touch them again while you are making measurements. The Telescope: It is used to observe the dispersed light. It can swing around the axis of the table and it has an angular scale attached to it so that the user can read its orientation (as described below). A screw across from the collimator allows you to secure the position of the telescope. With the position secured you can use a second knob just underneath the telescope to make fine adjustments to its position. 3.2. How to Read the Vernier Scale The main angular scale of the spectrometer is attached to the telescope and rotates as the telescope swings around. This scale is calibrated and numbered in degrees and it has small tickmarks at 30′ intervals. It can be read through a small, transparent window on the tabletop with the help of a magnifying glass. Right up against the main scale there is a vernier scale which is fixed to the tabletop. With the help of the vernier scale one can read the main angular scale to 1′ using the following procedure (refer to Figure 2). • Read the main angular scale against the zeroth tickmark of the vernier scale, truncating the result to the nearest 30′ (small tickmark). The “zeroth” tickmark of the vernier scale is in the direction of lower values of the main angular scale. For example, if the zeroth tickmark of the vernier scale is between 72◦ 30′ and 73◦ 00′ , you would read it as 72◦ 30′ . This example is illustrated in Figure 2. • Compare the tickmarks of the vernier scale with the tickmarks of the main angular scale. The vernier scale is such that it has 31 tickmarks in the same space that the main –3– Astro 452 Spectroscopy Fall 2004 angular scale has 30 tickmarks. As a result only one tickmark of the vernier scale can match a tickmark of the main angular scale at any given time. Look for the matching tickmarks and read the position along the vernier scale where the tickmarks match. This is the number of minutes of arc that you have to add to the above measurement. In the example of Figure 2 the 13th tickmark of the vernier scale matches up with a tickmark on the main angular scale (counting from right to left, starting from zero). This means that you should add 13′ to the coarse reading obtained above to get 72◦ 30′ +13′ =72◦ 43′ . This is the final measurement. Figure 2: – The angular vernier scale indicating an angle of 72◦ 43′ . Notice that only one of the tickmarks of the vernier scale matches up with a tickmark of the main angular scale. In this particular case it is the 13th tick mark of the vernier scale that matches up with a tickmark from the main angular scale (counting from right to left, starting from zero). 3.3. Dispersion of Light by a Grating Figure 3 shows schematically what happens to a monochromatic beam of light as it passes through a diffraction grating. It depicts the special case of an incident beam that makes a right angle with the plane of the grating. The original beam (left) is diffracted by each of the grooves of the grating and the resulting beams are made to interfere in the far field to give the pattern shown on the right. The final interference pattern consists of a number of spots in the directions shown by the arrows. The spots are labeled by their order, with n = 0 representing the beam that goes straight through, n = ±1 the beams on either side of that, and so on. The deflection angle (as defined in Figure 3) of the nth order beam, φn , is given by the grating equation d sin φn = nλ , (1) where d is the spacing between the grooves of the grating, n labels the order and λ is the wavelength of the light incident on the grating. When a grating is used as the dispersive device in the spectrometer it is mounted on the table so that the collimator can direct light to it and the telescope is used to view the dispersed light and measure deflection angles. –4– Astro 452 Spectroscopy Fall 2004 Figure 3: Dispersion of a monochromatic beam of light by a transmission grating. This is an illustration of the special case of a beam that hits the grating at right angles. Figure 4: Diffraction and interference of a monochromatic beam of light passing through a transmission grating. This is an illustration of the case that you will encounter in the lab, where the angle of incidence, i is non-zero. In practice, unless one carries out a careful alignment procedure, the incident beam will not hit the grating at right angles. As a result, the grating equation must be modified to include the angle of incidence of the beam (measured relative to the normal to the grating). Moreover, the angle of deflection after passing through the grating is not measured relative to the normal to the grating, as the grating equation assumes. Rather it is measured relative to the incident beam because it is fairly easy to align the telescope –5– Astro 452 Spectroscopy Fall 2004 with the collimator. This situation is depicted in Figure 4, which shows the convention used to measure angles in practice. This is the convention that you should assume for this experiment. In this convention the grating equation becomes d [sin i + sin(θn − i)] = nλ , (2) where θn is the deflection angle as you will be measuring it (see Figure 4) and i is the angle of incidence of the beam onto the grating. The angle of incidence is, in principle, unknown and it has to be measured after the grating is set up. Life becomes considerably easier, if the grating is approximately perpendicular to the incident beam, i.e., when the angle of incidence, i, is small (no more than a few degrees). In this case one can determine the value of i by measuring the deflection angle for light of known wavelength and using the formula sin i ≈ nλ − d sin θn d (1 − cos θn ) (3) This is a very important calibration step that you must carry out before measuring any unknown wavelengths with the grating. 3.4. Dispersion of Light by a Prism A monochromatic ray of light passing through a prism, as shown in Figure 5, suffers refraction twice and is deflected from its original direction. The deflection angle, θ, depends on the angle of incidence onto the face of the prism, the apex angle of the prism and the refractive index of the material of the prism. Since the refractive index is a function of wavelength, the deflection angle is also a function of wavelength. As a result a beam of white light passing through a prism is dispersed because the constituent monochromatic rays have a different deflection angle, according to their wavelengths. Figure 5: Path of a monochromatic ray of light through a prism. The apex angle of the prism is denoted by α and the angle of incidence is denoted by i. The angle between the original and final directions of the ray (the deflection angle) is denoted by θ . –6– Astro 452 Spectroscopy Fall 2004 The relation between the angle of incidence and angle of reflection and the properties of the prism allows one to determine the index of refraction at a given wavelength, µλ , as follows: sin2 i + sin2 (θλ + α − i) + 2 cos α sin i sin(θλ + α − i) 2 , (4) µλ = sin2 α where i and θλ are the angle of incidence of the original beam and the deflection angle of a ray of a particular wavelength, λ, respectively. The apex angle of the prism is denoted by α. This is the formula that you will be using to determine the refractive index of a glass prism in the lab by measuring the deflection angle for light at a few different wavelengths. In practice, the prism you will use has an apex angle of α = 60◦ , which on substitution in the above equation gives: µ2λ = 4 2 sin i + sin2 (θλ + α − i) + sin i sin(θλ + α − i) 3 (5) 4. Experimental Procedure The preceding sections give you all the information and tools you need to carry out the experimental procedure. The ultimate goal is to measure the refractive index of the glass of a prism at a few different wavelengths. The light sources that you will use in this experiment are Hydrogen, Helium, and Mercury lamps. These are glass tubes filled with gas which are mounted on a lamp holder. The lamp holder applies a very high voltage across the ends of the tube to produce an electrical discharge and make the gas inside the tube glow by emitting lines. SAFETY PRECAUTIONS You should be very careful when handling the lamps! In particular, you should take the following precautions: • The glass tubes are very delicate. Handle them with extreme care so that you do not break them. • The lamps get very very hot. Never touch them while they are on. • The lamp holder has a high voltage across its terminals. Never touch the terminals, especially not when the device is turned on. • To replace a lamp follow the steps below. Ask the instructor for help if you have any doubts, whatsoever. - turn the lamp holder off - wait for several minutes for the lamp to cool - remove the lamp carefully - install the new lamp carefully - turn the lamp holder back on. –7– Astro 452 Spectroscopy Fall 2004 The procedure is described below as a series of steps. There are questions scattered around in the text, which you should answer in your lab reports. There are two experimental exercises, each of which is meant to be carried out in a separate lab session. Exercise 1: Wavelengths of Helium Lines (a) Spectrometer Setup: Make sure that no dispersive device is mounted on the table of the spectrometer. Turn on the lamp and put it right against the collimator slit. Open up the slit to a width of seveal millimeters to let light through. Swing the telescope around and look right down the collimator at the slit. First, focus the telescope eyepeiece so that the crosshairs inside it are in focus. Then focus the collimator so that the image of the slit is in focus (the slit jaws should look very sharp). Close the slit to a millimeter or two and move the telescope so that the crosshairs are aligned with the right edge of the image of the slit. You should always line up the crosshairs this way when you are making any measurements. Make sure that the telescope is secured and use the fine adjustment knob to refine the alignment. Read the angle of the telescope off the vernier and take a note of it. This angle corresponds to the direction of no light deflection. All angles from now on should be measured with respect to this direction by subtracting this angle. (b) Grating Alignment and Calibration: Mount the grating holder on the table and put the grating on it. Adjust the height of the table so that the grating is in the beam and align the grating by eye so that it is as close as possible to being perpendicular to the beam from the collimator. Mount the hydrogen lamp on the lamp holder and put it up against the slit. You can use a piece of cardboard with a hole in it to block the glare from the lamp (the hole lets the collimator look at the lamp). By swinging the telescope around find the firstorder spectrum of the lamp; it should consist of a few spectral lines with bright colors. Adjust the position of the lamp so that the light goes right through the slit and adjust the slit width so that the lines are as sharp as possible (check the focus too). * How many emission lines can you see? What are their colors? * These are the lines of the Balmer series of Hydrogen, whose wavelengths you should have calculated before coming to the lab. Which line is which? Measure the deflection angle of as many lines as you can see. You should be able to see the same spectrum on the opposite side of the undeflected beam (at negative deflection angles). So, you should make duplicate measurements of all the lines. Using the deflection angle and wavelength of each line calculate the angle of incidence of the beam onto the grating by applying equation (3). The grating has 600 lines/mm . * Combine the results to get the best estimate of the angle incidence and its uncertainty. * After you have determined i you can check the assumption that it is a small angle (on which the equation you used is actually based). A small angle should have cos i ≈ 1. –8– Astro 452 Spectroscopy Fall 2004 (c) Measurement of Wavelengths of Helium Lines: Replace the Hydrogen lamp with the Helium lamp following the precautions given above. Observe the spectrum of the Helium lamp and desribe it. * How many lines can you see? How brght are they? What are their colors? * What is your best guess of the wavelengths based on the colors? Measure the deflection angles of the few brightest lines (make duplicate measurements, as above). Calculate the wavelength of each line using equation (2). Take the average of the duplicate measurements. Exercise 2: Refractive Index of Glass (a) Spectrometer Setup: Repeat the procedure described above. Since you are using a different spectrometer than in the previous exercise, it is not safe to assume that it has been set up correctly, so you should carry out the entire procedure carefully. (b) Setup and Alignment of the Prism Put the prism holder on the table. Mount the prism so that the apex points away from the holder and the face that is in the direction of the collimator is lined up with the center of the table (the center is marked with a set of grooves). The arrangement is shown in Figure 6. This process can be tedius so you will have to fiddle with it. You will probably need to remove one of the screws that holds the grating holder to the table so that it can swing around. The purpose of this mounting procedure is so that you can observe the beam that is reflected from the face of the beam with the telescope. This is necessary in order to measure the angle of incidence onto the face of the prism. Show the setup to the instructor to make sure that you have it right. Swing the telescope around and look for the reflected beam. If you have trouble finding it, you can use the small mirror provided to help you: put it right against the face of the prism to improve reflectivity and then remove it when you find the beam. Measure the deflection angle of the beam reflected off the face of the prism. * How is this deflection angle that you just measured related to the angle of incidence? * What is the angle of incidence? (c) Measurement of The Refractive Index of Glass The Hydrogen and Helium lamps provide a set of bright emission lines from Hydrogen and Helium whose wavelengths are known. These lines can serve as monochromatic light sources with which you can measure the refractive index of glass in the prism as a function of wavelength. The Hydrogen lines should be easy to identify and the wavelengths can be calculated as shown in §2 of this manual. As for the Helium lines, see the chart in Figure 7 at the end of this manual. Measure the deflection angle through the prism for as many bright lines as possible in the Hydrogen and Helium spectra with known wavelengths. Pick lines spanning the entire range between blue and red. For each of these lines use equation (5) to compute the refractive index of glass at that wavelength. The prism has an apex angle of α = 60◦ . –9– Astro 452 Spectroscopy Fall 2004 Figure 6: Position and alignment of the Prism onto the spectrometer table. The incident beam from the collimator and the beam reflected from the face of the prism are also shown. The refractive index of glass can be approximated by the Hartmann dispersion relation: B , µλ = A + λ−C where A, B, and C are known as the Hartmann constants. With measurements of the refractive index at 3 different wavelengths, the constants can be determined as follows (see Kitchin, §4.1, p.302, but beware of typo): λ1 (λ2 − λ3 )(µ1 − µ2 ) − λ3 (λ1 − λ2 )(µ2 − µ3 ) (λ2 − λ3 )(µ1 − µ2 ) − (λ1 − λ2 )(µ2 − µ3 ) −1 1 1 − B =(µ1 − µ2 ) λ1 − C λ2 − C B A =µ1 − λ1 − C C= * Use the measurements you just made to compute the Hartmann constants. * Kitchin (4th edn, §4.1, p.323) gives the Hartmann constants for two different types of class. Do the constants that you find match with either of those sets? If there is a discrepancy, what do you think is its source? * If you measured more than 3 spectral lines, use the first 3 to compute the Hartmann constants and then use the Hartmann dispersion relation to compute the other refractive indices that you have measured. Do the predictions agree with the measurements? * Make a plot of µλ vs λ and include it in your lab reports. – 10 – Astro 452 Spectroscopy Fall 2004 5. Lab Reports In your lab reports you should give a brief description of the method that you followed without getting into too much detail about the alignment procedures of the spectrometer. Make sure that you answer all of the above questions. An important part of your report should be the consideration of measurement uncertainties and how they can affect your results. Here are two particular issues you should consider, among others: 1. What is the effect of misreading the vernier scale by 1′ ? Starting from equation (2) in this manual derive an equation for the uncertainty in the measured wavelength in this particular experiment and evaluate it for a typical measurement. Is such an error a significant cause for concern? 2. What is the effect of the uncertainty in the angle of incidence of the beam onto the grating? Use equation (2) to derive an expression for the uncertainty in the wavelength that would result from an uncertainty in the angle of incidence. Evaluate it using the uncertainty in i that you got above. Is this a serious cause for concern? Figure 7: Spectrum of the He lamp, as one would see it through the spectrometer. The brightest lines are identified and labelled their wavelengths in Å. – 11 –