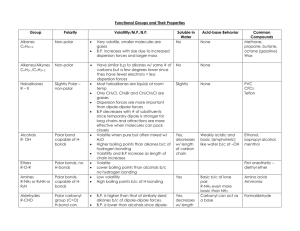
Intermolecular Forces: Boiling Point, Evaporation, Solubility
advertisement
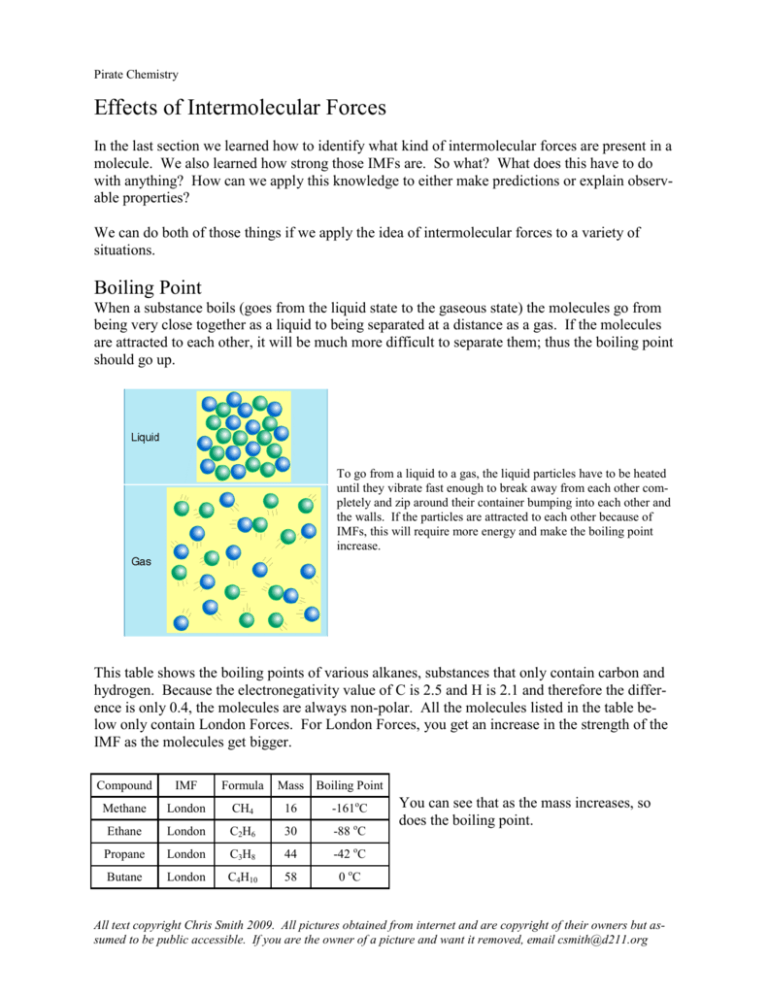
Pirate Chemistry Effects of Intermolecular Forces In the last section we learned how to identify what kind of intermolecular forces are present in a molecule. We also learned how strong those IMFs are. So what? What does this have to do with anything? How can we apply this knowledge to either make predictions or explain observable properties? We can do both of those things if we apply the idea of intermolecular forces to a variety of situations. Boiling Point When a substance boils (goes from the liquid state to the gaseous state) the molecules go from being very close together as a liquid to being separated at a distance as a gas. If the molecules are attracted to each other, it will be much more difficult to separate them; thus the boiling point should go up. To go from a liquid to a gas, the liquid particles have to be heated until they vibrate fast enough to break away from each other completely and zip around their container bumping into each other and the walls. If the particles are attracted to each other because of IMFs, this will require more energy and make the boiling point increase. This table shows the boiling points of various alkanes, substances that only contain carbon and hydrogen. Because the electronegativity value of C is 2.5 and H is 2.1 and therefore the difference is only 0.4, the molecules are always non-polar. All the molecules listed in the table below only contain London Forces. For London Forces, you get an increase in the strength of the IMF as the molecules get bigger. Compound IMF Formula Mass Boiling Point Methane London CH4 16 -161oC Ethane London C2H6 30 -88 oC Propane London C3H8 44 -42 oC Butane London C4H10 58 0 oC You can see that as the mass increases, so does the boiling point. All text copyright Chris Smith 2009. All pictures obtained from internet and are copyright of their owners but assumed to be public accessible. If you are the owner of a picture and want it removed, email csmith@d211.org Pirate Chemistry This next table shows similarly sized molecules. Thus they have roughly the same amount of London forces. However, the examples given show wildly different boiling points because the molecules exhibit different strength of intermolecular forces. Remember that: Strength of IMFs: London Forces < Dipole Forces < H-bonds Molecules with stronger IMFs tend to have higher boiling points because their intermolecular forces are stronger; the molecules ―stick‖ together stronger and are harder to pull apart. Compound IMF Formula Butane London C4H10 58 0 oC Propanal Dipole C3H6O 58 49 oC 60 97 oC Propanol H-Bonds C3H7OH Mass Boiling Point All of these molecules have almost identical masses. However, their boiling points are very different due to the variances in the types of IMFs present. H H H H H C C C C H H H HH H H O H C C+ C H H H Butane, C4H10 Weak London Forces Propanal, C3H6O Stronger Dipole Forces — H H H — H C C C O + H H H H Propanol, C3H7OH Strongest H-Bonds Here is another example of this phenomenon. Examine the two structures below. Both have 2 carbon atoms, 8 hydrogen atoms, and 1 oxygen atom. In the first case, ether, the oxygen is in the middle and is bonded to the carbon atoms. In the second case, ethanol, the oxygen is near the end and is directly bonded to a hydrogen atom. Ether is polar with Dipole Forces but the ethanol is polar with H-Bonds. Ether boils at –23 oC while Ethanol boils at 78.5 oC; over 100 o C more for simply re-arranging the atoms to get the capacity to form H-bonds! — H C H H O + H C H H Ether, H3COCH3 Dipole Forces Boiling Pt: -23 oC H H — H C C O + H H H Ethanol, C2H5OH H-bonds Boiling Pt: 78.5 oC Boiling Point increases as the strength of the intermolecular forces increases. Substances with stronger IMFs have higher boiling points. All text copyright Chris Smith 2009. All pictures obtained from internet and are copyright of their owners but assumed to be public accessible. If you are the owner of a picture and want it removed, email csmith@d211.org Pirate Chemistry Evaporation Similar to the boiling point situation, molecules that have strong intermolecular forces tend to evaporate slower than those with weak intermolecular forces. Look at the picture below. Two beakers were filled to the 100 mL mark; one with water and one with cyclohexane. Water is polar with strong H-bonds while cyclohexane is non-polar with only weak London Forces. The beakers were allowed to sit, side-by-side for a period of time. Examine the second picture. The water has hardly evaporated at all while the a considerable amount of the cyclohexane has evaporated in the same time period. Cyclohexane is held together weaker than water and thus the molecules evaporate faster. H — H O + H H H CH C H C H H C Water, H2O Strong H-bonds HC H C H H H Cyclohexane, C6H12 Weak London Forces cyclohexane water cyclohexane water After some time, the water has evaporated very little and the cyclohexane has evaporated more If the particles in a sample have stronger intermolecular forces, the liquid will evaporate slowly as the molecules are held together by their attractions. All text copyright Chris Smith 2009. All pictures obtained from internet and are copyright of their owners but assumed to be public accessible. If you are the owner of a picture and want it removed, email csmith@d211.org http://www.tapintoquality.com/facts/glossary/ Both liquids start at the same level here Pirate Chemistry Look at the pictures below for one more example. The first beaker contains ethanol C2H5OH, the second beaker contains ethylene glycol C2H4(OH)2, and the third beaker contains glycerol C3H5(OH)3. All three of these substances contain –OH groups which means they all make strong H-bonds. But ethanol has only 1 site to make these, ethylene glycol has 2, and glycerol has 3 sites to make H-bonds. Consequently, after a period of time, much of the 1st has evaporated, some of the 2nd has evaporated, and little of the third has evaporated. Glycerol has the strongest IMFs because it has the most sites for H-bonding and so evaporates the slowest. H H H C C +O H H H — Ethanol, C2H5OH 1 H-bond site — H H — O C C O + + H H H H Ethylene Glycol, C2H4(OH)2 2 H-bond sites — — H H H O +C +C C O + H H O H H — H Glycerol, C3H5(OH)3 3 H-bond sites Ethanol, C2H5OH 1 H-bond site Ethylene Glycol, C2H4(OH)2 2 H-bond sites Glycerol, C3H5(OH)3 3 H-bond sites All liquids start at the same level Ethanol, C2H5OH 1 H-bond site Evaporate fastest Ethylene Glycol, C2H4(OH)2 2 H-bond sites Evaporates slower Glycerol, C3H5(OH)3 3 H-bond sites Evaporates slowest Ethanol has evaporated most after some time Evaporation rate increases as the strength of the intermolecular forces decreases. Substances will stronger IMFs evaporate slower. All text copyright Chris Smith 2009. All pictures obtained from internet and are copyright of their owners but assumed to be public accessible. If you are the owner of a picture and want it removed, email csmith@d211.org Pirate Chemistry Solubility Let’s examine two very different substances, water and cyclohexane. We saw them earlier when we discussed evaporation. Water is very polar with H-bonds while cyclohexane is nonpolar with only London Forces. Let’s mix the two liquids together. Look what happens: The two liquids don’t mix. There are two distinct layers of liquids in the tube. Even if the tube is shaken and the two liquids are mixed up, they eventually separate back into their two layers. Water and cyclohexane shaken together to mix them up Two layers beginning to re-form What’s going on here? Why won’t they mix? All text copyright Chris Smith 2009. All pictures obtained from internet and are copyright of their owners but assumed to be public accessible. If you are the owner of a picture and want it removed, email csmith@d211.org Pirate Chemistry Obviously this must have something to do with their different intermolecular forces. Remember: H H O + H H H Water, H2O Strong H-bonds CH C H C H H C HC H C H http://wpcontent.answers.com/wikipedia/commons/ thumb/6/65/Cyclohexane-3D-space-filling.png/100pxCyclohexane-3D-space-filling.png — H H Cyclohexane, C6H12 Weak London Forces The polar water molecules are going to be attracted to each other. There is no reason for them to want to have any interactions with the cyclohexane. Even if the two were mixed up, the water molecules will ―find‖ each other in the mixture as their negative and positive charges attract to each other. This will leave the cyclohexane molecules to group together into their own layer which is why we see the two distinct layers. Even when forced to be mixed up, the polar water molecules will be attracted to each other and not the cyclohexane. The waters will begin to attract each other because of their polar sites and begin to group together Eventually all the water molecules will be together in one layer with the cyclohexanes in another layer. Non-polar layer Polar layer All text copyright Chris Smith 2009. All pictures obtained from internet and are copyright of their owners but assumed to be public accessible. If you are the owner of a picture and want it removed, email csmith@d211.org Pirate Chemistry http://www.stevespanglerscience.com/img/cache/bcb9b8db117ee64376aedaf7af3595ca/sevenlayer-251908.jpg Notice in the last picture, the cyclohexane is on top and the water is on the bottom. Why is that? Remember our density column? This picture shows four different liquids in what is called a density column. The bottom layer is the most dense and the top layer is the least dense. Water has a density of 1.0 g/mL while cyclohexane has a density of 0.78 g/mL. Cyclohexane is less dense so floats on top. Cyclohexane has a density of 0.78 g/mL. It floats on top. Water has a density of 1.0 g/mL. It sinks to the bottom. All text copyright Chris Smith 2009. All pictures obtained from internet and are copyright of their owners but assumed to be public accessible. If you are the owner of a picture and want it removed, email csmith@d211.org Pirate Chemistry The basic idea from the previous example is that molecules that are polar do not tend to mix with those that are non-polar. Polar and non-polar don’t mix. Implied in that statement is another idea, though. If polar and non-polar don’t mix, will polar mix with polar? Will non-polar mix with non-polar? Let’s do 2 quick experiments. Experiment 1 Let’s mix two different polar liquids together: water and ethanol. H H H C C +O H H H Ethanol, C2H5OH 1 H-bond site — — H O + H Water, H2O 2 H-bond sites Both of these liquids are polar with H-bonding capabilities. When they are mixed together here is the result: Water and ethanol Both mixed together gives only 1 layer Notice that there is only one layer here. The polar molecules of the two different liquids are perfectly happy to be attracted to each other because of their similar properties. What would happen if two non-polar liquids are mixed? All text copyright Chris Smith 2009. All pictures obtained from internet and are copyright of their owners but assumed to be public accessible. If you are the owner of a picture and want it removed, email csmith@d211.org Pirate Chemistry Experiment 2 Let’s mix two different non-polar liquids together: cyclohexane and toluene H H CH H C H C H H HC H C C H H H Cyclohexane, C6H12 Weak London Forces H H H C C C H C C C C H H H H Toluene, C6H5CH3 Weak London Forces Both of these liquids are non-polar with only London Forces. When they are mixed together here is the result: Cyclohexane and toluene Both mixed together gives only 1 layer Notice again that there is only one layer here. The non-polar molecules of the two different liquids are soluble (able to dissolve) in each because of similar properties. We can summarize this concept in a different way: Like dissolves like. Polar substances will dissolve in other polar substances and nonpolar substances will dissolve in other non-polar substances. All text copyright Chris Smith 2009. All pictures obtained from internet and are copyright of their owners but assumed to be public accessible. If you are the owner of a picture and want it removed, email csmith@d211.org Pirate Chemistry Questions 1. 2. 3. 4. What is the relationship between strength of intermolecular forces and boiling point? What is the relationship between strength of intermolecular forces and rate of evaporation? What is the rule that governs whether or not one substance will dissolve in another? List the following intermolecular forces in order of increasing strength: H-bonds London Forces Dipole Forces 5. Put the following substances in order of increasing boiling points: C2H6 C5H12 C6H14 CH4 C3H8 6. Which of the following substances will most like have the highest boiling point? C6H12 C6H11OH C4H10 C5H8(OH)3 7. Which of the following substances will most likely evaporate the fastest? C5H12 H2O C4H6O C2H5OH 8. Which of the following substances will most likely evaporate the slowest? C2H5OH C2H6 C2H4(OH)2 9. Which of the following substances would be most likely to dissolve in water? CH4 CH3OH C6H6 C6H11O 10. If you wanted to dissolve grease, a non-polar substance, off your hands, which of the following liquids would be best? Water, H2O Antifreeze, C2H4(OH)2 Gasoline, C8H18 Alcohol, C2H5OH 11. If substance A (density 0.95 g/mL) exhibits H-bonding and substance B (density 1.23 g/mL) has only London forces and the two were mixed into the beaker to the right. How many layers would form? Draw them. Label the layers as A or B. Temperature (Celcius) Boiling Point (deg Celcius) 12. Explain why water has such an unusually high boiling point relative to the other hydrides in group VI even though water is the smallest of the elements in the group. 150 100 50 0 -50 -100 Boiling Point (deg Celcius) H2O H2S H2Se H2Te 100 -60 -40 0 Substance Boiling Point (deg Celcius) All text copyright Chris Smith 2009. All pictures obtained from internet and are copyright of their owners but assumed to be public accessible. If you are the owner of a picture and want it removed, email csmith@d211.org