Overview of Chapter 13 Intermolecular Forces Dipole
advertisement

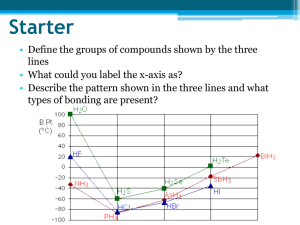
Overview of Chapter 13 • Intermolecular Forces • Liquids and their properties Intermolecular Forces Strongest to weakest Na+ Cl- • Ion-Ion • Ion- Dipole • Dipole-Dipole • Solids and their properties • Dipole-Induced dipole • Phase diagrams • Induced dipole-Induced dipole • Hydrogen Bonding (Dipole-Dipole) Dipole-Induced Dipole interactions Dipole-Dipole Forces Influence of dipole-dipole forces is seen in the boiling points of simple molecules. Compound N2 NH3 HF Mol. Wt. 28 14 20 Boiling Point -196°C -33°C 20°C N2 NH3 HF 0D 1.42 D 1.8 D Okay, so polar molecules can coalesce to form liquids, but what’s up with non-polar molecules? How can non-polar molecules such as O2 and I2 dissolve in water? The water dipole INDUCES a dipole in the O2 electric cloud. Induced dipole - Induced dipole Formation of a correlated dipole in two nonpolar molecules. i.e. Why is gasoline a liquid? Induced Dipole-Induced Dipole interactions! aka London dispersion forces Occurs in ALL molecules (all you need is electrons) 1 Boiling Points of Hydrocarbons Induced Dipole-Induced Dipole • Works with all molecule types • Works better with larger atoms and molecule (those are more polarizable) • These forces are weak, but they add up C4 H10 C3 H8 C2 H6 CH4 in vacuo Bulk H2O 1.85 D 2.9 D In general, BP increases with molecular weight The larger the molecule the more electron density More electron density yields a more polarizable molecule Polarity and Solubility Polarity and Solubility “like dissolves like” “like dissolves like” i.e. Polar substances mix well with other polar material Non-polar with nonpolar Oil and water don’t mix 1st: dissolve I2 in H2 O (dipole-induced dipole interaction) 2nd: layer I2 in H2O over CCl4 (polar - non-polar do not mix 3rd: shaking allows non-polar I2 to move to CCl4 Boiling Points of Simple Hydrogen-Containing Compounds Hydrogen Bonding A special form of dipole-dipole attraction, which enhances dipole-dipole attractions. 6A 5A 7A 4A H-bonding ONLY when X and Y are N, O, or F 2 Hydrogen Bonding Between Methanol and Water -δ Hydrogen Bonding in H2O H-bonding is especially strong in water because • the O— O— H bond is very polar • there are 2 lone pairs on the O atom • there are 2 H’ H’s available to bond H-bond +δ H-bonding accounts for many of water’ water’s unique properties. -δ Hydrogen Bonding in H2O Ice has open lattice-like structure. Hydrogen Bonding in H2O Ice has open lattice-like structure. Ice density is < liquid, so ice floats on water. One of the VERY few substances where solid is LESS DENSE than the liquid. What is this? What is this? 3


![[Answer Sheet] Theoretical Question 2](http://s3.studylib.net/store/data/007403021_1-89bc836a6d5cab10e5fd6b236172420d-300x300.png)