Workshop on Quantitative Pharmacology and Systems Biology in
advertisement

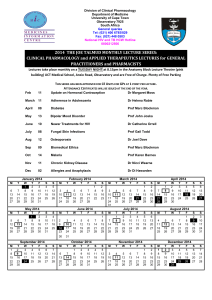
Workshop on Quantitative Pharmacology and Systems Biology in Pharmaceutical Development June 18-20, 2014 Dublin City University Workshop on Quantitative Pharmacology and Systems Biology in Pharmaceutical Development A workshop examining the background principles, application, and regulatory aspects of systems pharmacology methods in emerging therapeutic developments for diabetes and cancer. Location: Dublin City University Dates: June 18-20, 2014 http://tinyurl.com/Dublin2014PKPD With the support of the following organisations School of Pharmacy and Pharmaceu1cal Sciences Purpose: Pharmaceuticals form the basis of the majority of treatments in medicine. Pharmacokinetics (PK - how the body affects the drug) and pharmacodynamics (PD - how the drug affects the body) are quantitative sciences that represent cornerstones of developing new and efficacious disease treatments. A rigorous understanding of PK and PD is required by the drug approval agencies of most nations. The modern approach in the field of PK/PD is the development of models based on mechanisms of drug action and their alteration of physiological processes. This 2.5 day workshop will highlight the principles, techniques, and applications of PK/ PD modelling in drug discovery, development and regulatory approval. Modelling and simulation allow the optimal design, integration and quantitative interpretation of pharmacological endpoints that range from molecular biology to human responses and can greatly expedite the drug development process. Didactic lectures and recent applications of PK and PD in oncology and metabolic diseases will be presented by leading experts in the field. At the conclusion of this workshop, participants from laboratory, clinical, and regulatory environments will be conversant with relevant quantitative models that are applicable in many aspects of biomedical research to provide a predictive understanding of pharmacological responses to diverse drugs. The programme is designed to bring together clinicians, systems biologists, mathematicians, engineers, drug developers, bioinformatics researchers and regulatory bodies for interactive discussions focused on their fields. Day 1 Wednesday June 18th Intensive Introduction to Pharmacometrics; Applications to Metabolic Diseases Time Details 8.00am Registration opens, Helix Conference Centre, DCU 9.00 Pharmacometrics and Bioinformatics Analysis of Pharmacological Systems I Clynes, Straubinger, O’Connor Fáilte and Introduction 9:15-10:10 Prof. William Jusko SUNY, University at Buffalo General PK/PD Modelling Perspectives • • • • • Objectives of PK/PD modelling: The field and this workshop Comparison of key elements of PD versus PK modelling Basic tenets of PD: Capacity-limitation and turnover/homeostasis Array of mechanistic PK/PD models Complexities in PK/PD modelling 10:10-11:00 Prof. Don Mager SUNY, University at Buffalo Population PK/PD Modelling • • • • • PK/PD variability Nonlinear mixed effects modelling Common inter-subject variability and residual error models Covariates explaining inter-subject PK/PD variability Examples of population PK/PD in drug development and therapeutics Break Prof. Des Higgins Professor of Bioinformatics, University College Dublin Multivariate Analysis of Gene Expression and Proteomics Data Sets Lunch • Methods for analysing data from gene expression microaray, RNA-seq or high throughput proteomics experiments 11:00-11:30 11:30-12:20 12.20-1.30 Drug and Therapy Discovery in diabetes • • • • Insulin signalling and glucose transport defects in diabetes Contraction-mediated glucose disposal The interaction between glucose transport and gene expression Mitochondrial function in metabolic regulation 1:30-2:10 Dr. Donal O’Gorman Director, Centre for Preventive Medicine Dublin City University Insulin-dependent and –independent regulation of glucose disposal in human skeletal muscle 2:10-2:50 Prof. Jochen Prehn Professor of Physiology & Director, Centre for Systems Medicine Royal College of Surgeons in Ireland Development of computational modelling approaches to understand bioenergetics and apoptosis signalling at a systems level: towards novel prognostic tools 2:50-3:30 Prof. William Jusko Modelling Anti-Diabetic Effects 3:30-3:50 Break 3:50-4:40 Prof. Don Mager Modelling Combination Therapy in Diabetes • Translational modelling of exenatide pharmacokinetics • Diverse models of pharmacodynamic interactions • Combination model of metformin and sitagliptin 4:40-5:30 Prof. John Findlay National University of Ireland-Maynooth A new therapeutic approach to human insulin resistance and type 2 diabetes • Retinal binding protein and insulin resistance • Novel compounds that promote glucose transport • Understanding the mechanism of action • New computational modelling approaches to understand the role of glucose metabolism, mitochondria, and apoptosis signalling • • • • Models for glucose – insulin dynamics: minimal to majestic Diverse mechanisms and models for anti-diabetic drug effects Target-mediated PK/PD models for vildagliptin From basic to systems drug/disease models in diabetes Day 1 Thursday June 19th Pharmacometrics and Bioinformatics; Applications in Oncology Time Details Pharmacometrics and Bioinformatics Analysis of Pharmacological Systems II 9:00-9:55 Prof.Amin Rostami Professor of systems pharmacology - Manchester Pharmacy School Physiologically-based PK Modelling in Drug Development 9:55-10:50 Prof. William Jusko PK/PD Models in Pharmacogenomics 10:50-11:20 Break 11:20-12:15 Dr.Tapesh Santra Systems Biology Ireland - University College Dublin Predicting cancer subtype specific therapeutic targets by analyzing high-throughput heterogeneous datasets 12.15-1.30 Lunch • Applications of “systems biology” approach to prediction of drug absorption, distribution and elimination • Extrapolation of in vitro data to predict in vivo pharmacokinetics • Applications of PBPK in drug development and therapeutics, particularly endocrinology, drug abuse, and paediatric pharmacology • Relevance of basic tenets of PK/PD: Capacity-limitation and turnover/homeostasis • Modelling diverse genomic effects of corticosteroids • Connecting genomics (PG) and proteomics (PP) • Circadian rhythms in gene expression in various tissues • Small systems models for corticosteroid PK/PD/PG/PP • Mathematical and computational modelling to analyse the network structure of cell signalling. • Prediction of responses to perturbations (such as drugs), define the most sensitive points for interference by drugs, and analyse the specificity of signalling and adaptation processes. Drug and Therapy Discovery in Cancer 1:30-2:15 Prof. William Jusko Chemotherapy PK/PD Models • • • • Expectations of irreversible mechanisms Cell growth and cytotoxicity: profiles and data fitting Adding complexities: cell cycling, nonlinear cytotoxicity, resistance Models for tumor growth and inhibition 2:15-3:00 Prof. Robert Straubinger Professor of pharmaceutical sciences - SUNY, University at Buffalo Modelling Stromal disruption in pancreatic cancer • Development of computational approaches to understand and overcome sromal resistance-mediated pancreatic cancer treatment failure 3:00-3:30 Break 3:30-4:15 Dr. Peter O’Gorman Consultant Haematologist, Mater Misericordiae Dublin Predicting Drug Response in Patients with Multiple Myeloma • Individualisation of the selection process for current and emerging therapies for leukaemia and lymphoma 4:15-5:00 Prof. Don Mager Translational Systems Pharmacology Modelling of Combinatorial Chemotherapy • Integrated cell signalling pharmacodynamic model of anti-CD20-based regimens • Logic-based and dynamical modelling of bortezomib response in U266 cells and xenografts Day 3 Friday June 20th Biologicals; Drug Development, Regulatory Aspects and IndustryApplications Time Details Experimental and Systems Approaches in Biological Drug Development 9:00-9:55 9:55-10:40 Prof, Don Mager PK/PD Modelling of Biological Therapeutics Prof. Sathy V. Balu-Iyer SUNY, University at Buffalo Role of Immunogenicity in Biological Drug Development • Characteristics and complexities of biological drug absorption and disposition • Target-mediated drug disposition • Role of FcRn in monoclonal antibody pharmacokinetics • Mechanisms of immunogenicity of protein drugs • Trace protein and antibody aggregates as immunogenic species • Novel approaches to limiting immunogenicity and inducing tolerance 10:40-11:00 Break 11:00-11:45 Dr. Juan Jose Perez-Ruixo Role of Quantitative Pharmacology in Biologic Drug Development • EPO, Peg-EPO, Romiplostim • Diverse examples of integrated PK/PD models of biological therapeutics, including oncology and bone disorders 11:45-12:10 Dr. Adrian Dunne Scientific director and Research Fellow, Model Based Drug Development group, Janssen Research and Development (Janssen Pharmaceuticals) • Introduction to population PK/PD, the industry perspective 12:10- 12.40 Dr. An Vermeulen Senior Scientific director, Johnson and Johnson • Industrial applications of population PK/PD in drug development 12:40-12.50 Closing Remarks - Martin Clynes (Director NICB) For nearby hotel accommodation We suggest the Crowne Plaza Santry: http://tinyurl.com/CPsantry or Holiday Inn Express, Dublin Airport: http://tinyurl.com/HIsantry Both are < 4km away Or accommodation on the DCU campus (http://www.summeraccommodation.dcu.ie/) We are grateful for support from the following sponsors Organising committee Speakers • Dr. Robert O’Connor, DCU, Senior Programme Leader Translational Cancer Pharmacology Dublin City University http://www.nicb.dcu.ie/staff_robert_o_connor.html • WJ Jusko, PhD, University at Buffalo, State University of New York, SUNY Distinguished Professor and Chair, Dept. of Pharmaceutical Sciences http://pharmacy.buffalo.edu/pages/176/Dr.-William-J.-Jusko.html • Prof. Martin Clynes, DCU, Director, National Inst. for Cellular Biotechnology Dublin City University http://www.nicb.dcu.ie/staff_martin_clynes.html • DE Mager, PharmD, PhD, University at Buffalo, State University of New York, Associate Professor, Dept. of Pharmaceutical Sciences http://www.pharmacy.buffalo.edu/pages/183/Dr.-Donald-E.-Mager.html • Prof. Robert M. Straubinger, University at Buffalo, State University of New York, Dept. of Pharmaceutical Sciences http://www.pharmacy.buffalo.edu/pages/183/Dr.-Donald-E.Mager.html • Prof. Donald E. Mager, PhD, PharmD, University at Buffalo, State University of New York, Dept. of Pharmaceutical Sciences http://pharmacy.buffalo.edu/phc/faculty/MAGER.HTML • Prof. Walter Kolch, UCD Conway, Director, Systems Biology Ireland http://www.ucd.ie/sbi/peoplepartners/profiles/walterkolch/ • Prof. Jochen Prehn, Head of Dept and Director Centre for Human Proteomics and Medical Systems Biology RCSI Pharmaceutical Sciences http://www.rcsi.ie/index.jsp?a=1759&n=613 • Dr. Donal O’Gorman, DCU, Director, Centre for Preventive Medicine, Health and Human Performance http://www.dcu.ie/info/staff_member.php?id_no=1485 • SV Balu-Iyer, PhD, University at Buffalo, State University of New York, Professor, Dept. of Pharmaceutical Sciences http://pharmacy.buffalo.edu/phc/faculty/BALU.HTML • Dr. Tapesh Santra, UCD Conway, Director, Systems Biology Ireland http://www.ucd.ie/research/people/systemsbiologyireland/drtapeshsantra/ • Prof. Des Higgins, UCD Conway, Professor of Bioinformatics http://www.ucd.ie/sbi/peoplepartners/profiles/deshiggins/ • Prof. Jochen Prehn, Head of Dept and Director Centre for Human Proteomics and Medical Systems Biology RCSI Pharmaceutical Sciences http://www.rcsi.ie/index.jsp?a=1759&n=613 • Dr. Donal O’Gorman, Director of the Centre for Preventive Medicine (CPM) at Dublin City University (DCU) http://www.dcu.ie/info/staff_member.php?id_no=1485 • Dr. Peter O’Gorman Consultant Haematologist, Mater Hospital, Dublin https://www.materprivate.ie/consultant/dr-peter-o-gorman/ • Dr. Juan Jose Perez-Ruixo Scientific Director at Amgen, Spain http://www.linkedin.com/profile/view?id=13185877&authType=OUT_OF_NETWORK&auth Token=IfTN&locale=en_US&srchid=155540931391630659760&srchindex=4&srchtotal=168&trk=vsrp _people_res_photo&trkInfo=VSRPsearchId%3A155540931391630659760%2CVSRPtargetId% 3A13185877%2CVSRPcmpt%3Aprimary • Prof. Amin Rostami, Professor of Systems Pharmacology, University of Manchester http://www.pharmacy.manchester.ac.uk/staff/Amin.Rostami • Prof. John Findlay, NUI Maynooth https://www.nuim.ie/people/john-findlay • Dr. Adrian Dunne, Scientific director and Research Fellow, Model Based Drug Development group, Janssen Research and Development • Dr. An Vermeulen, Senior Scientific director, Johnson and Johnson • Prof. Robert M. Straubinger, University at Buffalo, State University of New York, Dept. of Pharmaceutical Sciences http://pharmacy.buffalo.edu/sopps/pages/564/Profile-Directory-Detail.html?ID=193