11157 - MIA AN - Blk
5/7/05
11:31 am
Page 2
Part of GE Healthcare
Application Note 11-0036-52 AA
IN Cell Developer Toolbox
Single-color mitotic index analysis using the IN Cell
Developer Toolbox
Key words: cell division mitotic index fluorescent nuclear dyes single-color image
analysis IN Cell Developer Toolbox
Cell division presents an interest to many areas of drug
discovery both as a specific target and as an application
for testing the effects of newly discovered drugs. The
mitotic index of a sample reflects the level of cell division
within a sampled cell population and is calculated as a
ratio of cells undergoing mitosis to the total number of
cells in the population.
A. Original nuclei image
B. All nuclei identified
In cells stained with fluorescent nuclear dyes, mitosis is
characterized by changes in fluorescence intensity and
distribution, which are indicative of doubling of DNA
content and chromatin condensation. These changes can
be analyzed using the IN Cell Developer Toolbox, a flexible
automated image analysis software package offering a
wide choice of functions and parameters for developing
user-defined analysis protocols.
Based on intensity- and morphology-related
characteristics of mitotic nuclei, image analysis with the
IN Cell Developer Toolbox allows identification of mitotic
nuclei in images of cells stained with a single fluorescent
DNA dye (Fig 1). Single-color analysis can be performed
purely on the basis of DNA quantity (fluorescence
intensity) and compactness of the nuclear material
(nuclear shape). This approach employs a simple assay
procedure that can be performed in either live cells (e.g.
using DRAQ5™) or fixed cells (using any DNA dye) and
correlates well with multi-step methods based on mitosisspecific antibody staining.
C. Mitotic nuclei (red) identified
Fig 1. Identification of mitotic nuclei in fixed cells using IN Cell Developer
Toolbox analysis software. Nuclear DNA was stained with DAPI. Images
were acquired on IN Cell Analyzer 1000.
11157 - MIA AN - Blk
5/7/05
11:31 am
Page 3
Materials
Products used
IN Cell Analyzer 1000
25-8010-26
N Cell Developer Toolbox Seat License
25-8098-26
Other materials required
Nocodazole (Sigma)
Anti-phospho-histone H3 Antibody (Upstate)
Alexa Fluor™ 488-anti-rabbit IgG antibody solution
(Molecular Probes)
4', 6-diamidino-2-phenylindole, DAPI (Sigma)
Single-color assay procedure
Before imaging, add the nuclear dye (e.g., DAPI, Hoechst™, or
DRAQ5) to the sample and incubate for 15 min. Cells may be
imaged fixed or live.
Single-color analysis procedure
1. Open the image stack in the IN Cell Developer Toolbox.
2. Create target set: ‘Nuclei’
a. Select channel with the image of the nuclei
marker (Fig 2).
5. Generate a User Defined Measurement to calculate
mitotic index as follows:
[Count<Count:\nuc_link\Nuclei>]/[Count<Count:\Nuclei>].
Here mitotic index is quantitated as the ratio of ‘linked
nuclei’ (i.e., the number of identified ‘Nuclei’ that are
linked to ‘Mitotic nuclei’) to the total nuclei number. Select
Statistical Function – Count for this measure to report
population summary data.
6. Export Summary table. Analyze data.
* The choice of a One to Many link can be explained as follows.
Late stages of mitosis involve separation of nuclear material as a
preface to the formation of the two daughter cells. In images of
nuclei labeled with fluorescent DNA dyes, this event results in the
appearance of two brightly stained objects within one nucleus. As
a result, when the intensity threshold is set quite high to separate
bright objects from other objects and background in the image
(for segmentation of the ‘Mitotic nuclei’ target set), not one but
two objects (sister chromatids) are identified in the cells
undergoing later stages of mitosis. Therefore, direct counting of
identified mitotic nuclei can lead to counting two chromatids
(within one mitotic nucleus) as two separate nuclei. To avoid this
artifact, we can link each ‘Nuclei’ target to corresponding ‘Mitotic
nuclei’ targets, allowing one nucleus to be linked with one or two
identified ‘Mitotic nuclei’ targets. Calculating the number of links
(i.e., counting how many nuclei have mitotic nuclei within them),
rather than performing a direct mitotic nuclei count, prevents
overestimation of the number of mitotic nuclei.
b. Select Filled option in the Target Details panel.
c. Select Preprocessing operations if required.
d. Select Segmentation option most appropriate for
an accurate nuclear segmentation (Fig 3).
e. Use Erode, Dilate, and Sieve Postprocessing
operations to refine nuclei bitmap (Figs 4 and 5).
3. Create target set: ‘Mitotic nuclei’.
a. Select channel with the image of the nuclei
marker (Fig 6).
Analysis flowchart
Image Source: Nuclear marker image
Target set:
Nuclei
Target set:
Mitotic nuclei
Preprocessing
Preprocessing
Segmentation
Segmentation
Postprocessing:
Erode, Dilate, Sieve
Postprocessing:
Erode, Sieve
b. Select Filled option in the Target Details panel.
c. Select Preprocessing operations if required
(which would be the same as for the first target set).
d. Select Segmentation option that will identify compact
nuclear region (Fig 7).
e. Use Erode if required. Use Sieve Postprocessing
operation to filter out debris (Figs 8 and 9).
f. Apply Acceptance Criteria based on intensity and
area to select bright compact nuclei only
(mitotic nuclei) (Fig 9).
4. Create a linked One to Many* target set (‘nuc_link’) to
link ‘Nuclei’ (Primary target set) with ‘Mitotic nuclei’
(Secondary target set). Set Overlap conditions as
required (for example, 70% overlap – secondary target
within primary target). Do not choose Allow multiple
primary targets to share secondary targets option.
2
Application Note 11-0036-52 AA 2005-05
Application of
Acceptance Criteria
Target link:
Nuclei-Mitotic nuclei
Measure mitotic index:
a ratio of mitotic nuclei count to all nuclei count
11157 - MIA AN - Blk
5/7/05
11:31 am
Page 4
Processing steps
‘Nuclei’ target set
‘Mitotic nuclei’ target set
Comparison of single-color and
dual-color analysis methods†
Single-color analysis of mitotic nuclei using the IN Cell
Developer Toolbox was compared with a conventional
dual-color method that employs specific antiphospho-histone H3 antibody staining. Statistical
analysis of the mitotic index data obtained by these
two different methods was then performed using
Pearson’s correlation test.
Method
Fig 2. Original nuclei image
Fig 3. Segmentation
Fig 6. Original nuclei image.
Fig 7. Segmentation
Hela cells were incubated with seven different
concentrations of nocodazole (0–3000 nM, 6 replicates
for each condition) for 18 h at 37 °C, 5% CO2.
Nocodazole acts by destabilizing microtubules and
arresting cells in mitosis.
Cells were fixed in methanol and, after washing with
PBS, incubated with 1% BSA/PBS for 30 min. The
blocking solution was then discarded and cells were
incubated with anti-phospho-histone H3 antibody
(1:100 in 1% BSA/PBS) for 90 min at room
temperature. This was followed by a PBS wash and
incubation for 90 min with secondary Alexa Fluor
488-anti-rabbit IgG antibody solution (1:1000 in 1%
BSA/PBS) containing 10 µg/mL of DAPI. After
incubation, cells were washed with PBS and the plate
imaged on the IN Cell Analyzer 1000.
Data from three replicate experiments were
compared using Pearson’s correlation test.
Results
Images were analyzed in the IN Cell Developer
Toolbox using two methods.
Fig 4. Postprocessing: Erosion
Fig 8. Postprocessing: Sieve
1. Analysis based on DAPI image only: Identifying
all nuclei and distinguishing mitotic nuclei among
them, based on the nuclear intensity- and
shape–related parameters.
2. Analysis based on Alexa Fluor and DAPI images:
Identifying mitotic nuclei in the Alexa Fluor image
and all nuclei in the DAPI image.
Fig 5. Postprocessing: Sieve and
Filled.
All nuclei identified (red).
Fig 9. Postprocessing: Sieve and
Filled and application of
Acceptance Criteria (based on
intensity and area).
Mitotic nuclei identified (red)
A representative dose-response graph from the
described experiment is shown in Figure 10. Data
from three experiments were compared using
Pearson’s correlation test. Test results demonstrated
statistically significant correlation of data obtained by
one- and two-color methods for mitotic index analysis
(Pearson correlation coefficient: r > 0.9, r2 > 0.81, and
P < 0.01 in three independent experiments).
3
Application Note 11-0036-52 AA 2005-05
11157 - MIA AN - Blk
5/7/05
11:30 am
Page 1
any DNA dye. Statistical analysis demonstrated significant
correlation of mitotic index data obtained by two different
analysis methods either using just nuclear dye images or
sets of two images containing both the nuclear dye DAPI
and Alexa Fluor-labeled anti-phospho-histone H3
antibody staining.
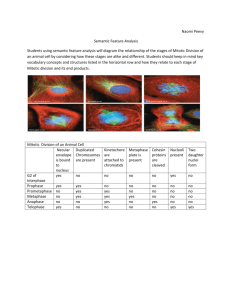
Fig 10. Typical result showing an increase in the number of mitotic nuclei in
response to nocodazole treatment. Mitotic nuclei were identified by two
methods: a traditional two-color method using Alexa Fluor-labeled antiphospho-histone-H3 antibody and DAPI (blue graph), and a single-color
method using nuclear dye DAPI only (red graph). Images were taken on IN
Cell Analyzer 1000 and analyzed using IN Cell Developer Toolbox.
Conclusion
While specific antibody-based dual-color analysis
provides accurate identification of mitotic nuclei, it is
usually limited to certain mitotic phases (antibody used in
the presented study labels mitotic nuclei before
anaphase). It is also restricted to working with fixed cells
only and involves a complex multi-step assay procedure.
Using IN Cell Developer Toolbox for analysis of the mitotic
index assay eliminates the need for specific antibody
staining thus simplifying the assay protocol and
preventing possible loss of M-phase cells due to the
multiple washing steps required for the antibody-based
method.
The mitotic index for a population of cells can be calculated
from analysis of single-color fluorescent nuclear dye images
using IN Cell Developer Toolbox. Images can be either
acquired from live cells (e.g., using DRAQ5) or fixed cells using
†
Asia Pacific
Tel: +852 2811 8693
Fax: +852 2811 5251
Latin America
Tel: +55 11 3933 7300
Fax: + 55 11 3933 7304
Australasia
Tel: + 61 2 9899 0999
Fax: +61 2 9899 7511
Middle East & Africa
Tel: +30 210 9600 687
Fax: +30 210 9600 693
Austria
Tel: 01/57606-1619
Fax: 01/57606-1627
Netherlands
Tel: 0165 580 410
Fax: 0165 580 401
Belgium
Tel: 0800 73 888
Fax: 03 272 1637
Norway
Tel: 815 65 555
Fax: 815 65 666
Canada
Tel: 1 800 463 5800
Fax: 1 800 567 1008
Portugal
Tel: 21 417 7035
Fax: 21 417 3184
Central, East, &
South East Europe
Tel: +43 1 982 3826
Fax: +43 1 985 8327
Russia & other
C.I.S. & N.I.S
Tel: +7 (095) 232 0250, 956 1137
Fax: +7 (095) 230 6377
Denmark
Tel: 45 16 2400
Fax: 45 16 2424
South East Asia
Tel: 60 3 8024 2080
Fax: 60 3 8024 2090
Finland & Baltics
Tel: +358-(0)9-512 39 40
Fax: +358 (0)9 512 39 439
Spain
Tel: 93 594 49 50
Fax: 93 594 49 55
France
Tel: 01 6935 6700
Fax: 01 6941 9677
Sweden
Tel: 018 612 1900
Fax: 018 612 1910
Germany
Tel: 0761/4903-490
Fax: 0761/4903-405
Switzerland
Tel: 01 8028 12
Fax: 01 8028 13
Italy
Tel: 02 27322 1
Fax: 02 27302 212
UK
Tel: 0800 616928
Fax: 0800 616927
Japan
Tel: +81 3 5331 9336
Fax: +81 3 5331 9370
USA
Tel: +1 800 526 3593
Fax: +1 877 295 8102
www.amershambiosciences.com/incell
GE Healthcare Ltd
Amersham Place
Little Chalfont
Buckinghamshire
HP7 9NA
UK
Images and data are taken from a collaborative study with
Banyu Pharmaceutical Co., Ltd., a subsidiary of Merck & Co.,
Inc. (Kyosuke Haze and Takayoshi Okabe, Japan).
General Electric Company reserves the right, subject to any
regulatory approval if required, to make changes in specifications
and features shown herein, or discontinue the product described
at any time without notice or obligation. Contact your GE
Representative for the most current information. © 2005 General
Electric Company - All rights reserved. GE and GE Monogram are
trademarks of General Electric Company. Amersham and
Amersham Biosciences are trademarks of GE Healthcare Limited.
DRAQ5 is a trademark of Biostatus Limited; Hoechst is a
trademark of Hoechst AG, and Alexa Fluor is a trademark of
Molecular Probes, Inc. The In Cell Developer Toolbox is the subject
of US patent application number 11/019326 in the name of
Amersham Biosciences Niagara, Inc. The IN Cell 1000 is the
subject of US patents 6,563,653 and 6,345,115 and US patent
application number 10/514925, together with other granted and
pending family members, in the name of Amersham Biosciences
Niagara, Inc.
GE imagination at work
Application Note 11-0036-52 AA 2005-05
4