SAM Teachers Guide Cellular Respiration - RI
advertisement

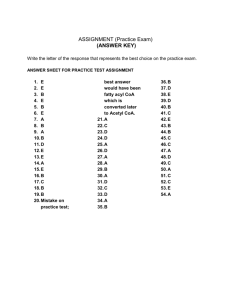
SAM Teachers Guide Cellular Respiration Overview Students explore the process of cellular respiration by studying the breakdown of glucose molecules as they pass from glycolysis, to the Krebs cycle, and the electron transport chain, leading to the production of ATP. In both glycolysis and the Krebs cycle the reactions are broken down into simple steps with 3D molecular views that allow students to follow the atoms as they rearrange and become parts of other molecules. The first part of the activity focuses on the production of high energy molecules that will later be used in the electron transport chain to create an imbalance of protons across the inner mitochondrial membrane. The second part of the activity allow the students to see the dynamic process of the electron transport chain in the production of this concentration gradient that will fuel ATP production at a later point. It is important to note, that it is not expected that students will memorize every step of glycolysis, the Krebs cycle, or the electron transport chain. The goal is to have them get the big picture of the types of reactions, that enzymes are important, and that energy in one form is converted or transferred to other forms and places. Learning Objectives Students will be able to: • Identify the steps in glycolysis and the Krebs cycle where ATP and NADH are produced and consumed. • Explain how the decrease in electron energy is linked to an increase in potential energy across the inner mitochondrial membrane. • Describe role protein complexes play in the electron transport chain as modes for electron transfer from one molecule to another and/or protons from one side of the membrane to the other. • Explain how the proton gradient created by the electron transport chain is then converted into the chemical energy stored in the form of ATP. Possible Student Pre/Misconceptions • That the energy produced by one process is not linked to energy reduced somewhere else. OJen conservation of energy is overlooked or misunderstood. • That the electron transport chain smoothly moves electrons from one place to another in some kind of directed fashion, which is a common interpretation of the static diagrams illustrating the process. Models to Highlight and Possible Discussion Questions ALer completion of Part 1 of the activity: Models to Highlight: • Page 1 – ATP molecule o Make connection to ATP + H20 ADP + Pi reaction o Explain in more detail how weak bonds have more chemical energy. When the phosphate bond is broken and then bonded to something else it can form a stronger bond converting the chemical energy into some other form of energy (oJen mechanical energy when a molecule changes shape due to phosphorylation.) • Page 3 – Glycolysis (with enzymes) o Point out how enzymes are necessary in each step. o Highlight the using up of ATP in step 1 and the creation of ATP in steps 7 and 10. o Make sure students realize that all reactions aJer step 5 happen twice. o Point out how NADH is also created and will be used later. • Page 4 – Krebs cycle o Highlight that the products of glycolysis then move into the mitochondria. o Point out how enzymes are needed here as well (and in almost every other biochemical reaction). o Point out NADH, the reaction where succinate becomes fumarate, as these will be part of the electron transport chain. Possible Discussion Questions: • Where do glycolysis and the Krebs cycle take place? • Why are mitochondria needed in almost every cell? • How much ATP is potentially produced by the reactions we have studied? How do you know? • What is the role of enzymes in biochemical reactions? ALer completion of Part 2 of the activity: Models to Highlight: • Page 6 – Exploring the Electron Transport Chain o Be sure to point out how the representation of the electron changes with the energy it posses. Higher energy means a blue star with more points. o This model can be very overwhelming, because there is a lot going on. However, this makes it especially rich for class discussion. You • can highlight only one protein complex at a time by clicking on it. This will be very helpful in reducing the amount of visual information students have to process. You may even want to go over this with them first, then let them observe for a while on their own, and then come back together. o There are a number of things to help students see here: • That the oxidation of a substance (loss of electron) is always associated with the reduction (gain of an electron) by another. • When electrons lose energy (change to a less pointed shape) the energy is used to pump protons (H+ ions) across the membrane, storing energy due to the concentration difference. • The protons are colored by their starting location, so it is easy to see aJer the model has run for a while that they are being pumped from the matrix (pink) to the inter‑membrane space (blue). • Point out the link with the Krebs cycle, both how the NADH produced in the cycle is used here, and that the 5th step of the cycle actually occurs at complex II in the membrane. • Help them see that NADH the lowest energy electrons end up in the H‑O bonds in water in complex IV. Page 8 ‑ Creating ATP with ATP Synthase o While there is not a model on this page it may be useful to show the linked video on this page and help students connect the electron transport chain process with how the proton gradient is then used to produce ATP. Possible Discussion Questions: • Give an overview of how the chemical energy in glucose is eventually converted to the chemical energy in ATP. • What happens to the high energy of the electrons that start out as part of NADH? The energy can’t just disappear. • Several substances are poisonous because they interfere with the electron transport chain. a) Why is the ETC important? b) Come up with one way the poison might specifically work (where/how might it disrupt the ETC)? Connections to Other SAM Activities The focus of this activity is for students to understand glycolysis and the production of ATP through the electron transport chain. This activity is supported by activities across physics, chemistry, and biology. From physics we get the conservation of energy in Atoms and Energy, and the ideas of electrical energy from concentrated charges in the Electricity activity. From chemistry an understanding reactions and the idea that specific amounts of glucose and ATP are involved comes from Chemical Reactions and Stoichiometry. The notion that energy is involved in reactions is explored in Chemical Reactions and Energy. Enzymes and protein complexes are the heart of the engine that generates ATP, so Four Levels of Protein Structure and Protein Partnering and Function are supporting activities from biology. Diffusion, Osmosis, and Active Transport is supported by Cellular Respiration because the ATP produced is used in the active transport section of the previous activity, showing how the chemical energy of ATP can be used to force ions to “diffuse” against their concentration gradient (from low concentration areas to high). Finally, Photosynthesis is supported by Cellular Respiration because glucose (the starting material for the ETC) is produced by photosynthesis. Activity Answer Guide Page 1: 1. How many phosphate groups are part of an ATP molecule? (c) 2. Why does the ATP molecule have a lot of chemical energy? (b) Page 2: 1. Glycolysis uses up some ATP (when it is a reactant) and makes some ATP (when it is a product). Overall, glycolysis makes more ATP than it uses. How much ATP is made overall? Take the total ATP produced and subtract the ATP used. (b) 2. The high energy molecule NADH is also created during glycolysis. The energy in these molecules will be used to make more ATP later. How many NADH molecules are produced during glycolysis? (b) 3. Each NADH molecule produced in glycolysis eventually is used to produced 2 ATP molecules. Given that some ATP is used up, some ATP is produced, and some NADH is produced that eventually becomes ATP, how much ATP overall is produced through glycolysis. (c) This shows glucose in the active site of hexokinase. There are many other possible answers. Settings to help highlight this were: “highlight changes”, “cartoon”, and “active site.” There are other molecules or ways to configure the image that would satisfy the answer to this question. 2. Take a snapshot, and place an image here that shows a PRODUCT in the active site of an enzyme. Make sure the product is clearly visible. 4. Glycolysis uses up some high energy molecules and makes some high energy molecules. Overall, does glycolysis use up energy or make more available. How do you know? It makes more high energy molecules than it uses. Only two ATPs are used up, and 4 are created. NADH is also created, which will be used to make more ATP later. Page 3: 1. Take a snapshot, and place an image here that shows a REACTANT in the active site of an enzyme. Make sure the reactant is clearly visible. Sample Picture: This shows 3-phosphoglycerate in the active site of phosphocycerate kinase. There are many other possible answers. Settings to help highlight this were: “highlight changes”, “cartoon”, and “active site.” There are other molecules or ways to configure the image that would satisfy the answer to this question. Page 4: 2. Place an image of FAD here: 1. How many high energy NADH molecules are produced for each pyruvate in the reactions above? (c) 2. How many high energy GTP molecules are produced for each pyruvate in the reactions above? (a) 3. How many high energy FADH2 molecules are produced for each pyruvate in the reactions above? (a) 4. Assume that each NADH will produce 3 ATP, each GTP will produce 1 ATP, and each FADH2 will produce 2 ATP. How many total ATP can be produced by the high energy molecules created in the Krebs cycle for each pyruvate that enters it? (b) 5. We have seen that glycolysis produces 6 ATPs in the making of two pyruvate molecules from glucose. You have also calculated how many ATPs are produced for each pyruvate that enters the Krebs cycle. How many TOTAL ATPs will be created by breaking down one glucose molecule? (c) 3. Place an image of succinate here: Page 5: 1. Place an image of NADH here: Note this is the same as the previous one because it contains both succinate and FAD. Page 6: This is one of three possible images they could use. 1. Which molecule is carrying electrons with the highest energy? (c) 2. Which molecule is carrying electrons with the lowest energy? (a) 3. Capture an image and highlight where cyanide blocks the electron transport chain. 3. Capture an image showing the process of how the decrease in electron energy is converted into an increase in the energy from concentration differences. Annotate your image to highlight important features. Cyanide blocks oxygen from binding to protein complex IV. In this image the electrons on NADH are losing energy as they are transferred to UQ. This energy is used to move two H+ ions (protons) from the matrix to the intermembrane space. Images capturing similar things on complex III and IV are also possible. 4. Pick one protein complex and describe in detail what is happening in your own words. There are four possibilities for an answer to this one. The four answers should be something like what is shown when you click on each of the purple protein complexes. 5. What is happening to the concentration of hydrogen ions (protons) on both sides of the membrane? (e) 4. Explain in detail why cyanide causes death (or serious illness in smaller doses). Cyanide blocks oxygen from binding to complex 4 so this shuts down the electron transport chain and your body stops making ATP. Soon you will run out of energy to keep your body functioning. Page 8: 1. The Electron Transport Chain creates potential energy by pushing protons to one side of the membrane, increasing the concentration on that side. What eventually happens to that energy? Explain in detail. That energy is converted to the chemical energy of ATP as the protons move through ATP synthase. 6. Describe the connection between the changes in hydrogen ion concentration and the changes in energy due to those concentration changes. As the hydrogen ion concentration increases in the intermembrane space (and decreases in the matrix) the energy increases. 2. One kind of poison not discussed on the previous page is a molecule that allows protons to diffuse through the membrane. Why is that a bad thing? If protons could diffuse through the membrane, then you could never build up a higher concentration of protons on one side and won’t be able to create an energy source for making ATP. Page 7: Page 9: 1. Which one of these DOESN'T completely shut down the Electron Transport Chain? (c) 2. Explain how rotenone affects the Electron Transport Chain. Rotenone block the transfer of electrons from NADH to UQ on protein complex I. 1. Which of the following statments is true regarding the ATP moleucle? (CHECK ALL THAT APPLY) (a, c, d) 2. Which one of the following statements is true regarding energy flow in biological systems? (b) 3. Which statement is true of enzymes? (a) 4. What role does the Krebs cycle play in the making of ATP? The Krebs cycle breaks down glucose, a common chemical in food, to create molecules with high energy electrons. The energy in those electrons is eventually used to create ATP. 5. Give an overview of what happens in the Electron Transport Chain and how this leads to the production of ATP. High energy electrons from molecules made in the Krebs cycle are transferred from one molecule to another, often losing energy in the process. That energy is used to pump protons (hydrogen ions) across a membrane, causing an increase in the concentration difference between the two sides of the membrane. This energy is then later used to cause ATP synthase to make ATP as the protons flow back through ATP synthase to restore the concentration balance. SAM HOMEWORK QUESTIONS Cellular Respiration Directions: After completing the unit, answer the following questions to review. Use the following image showing one step in glycolysis to answer questions 1-2: 1. In the chemical reaction above, what is the name of the enzyme? 2. Is this a step in glycolysis that makes ATP or uses it up? Explain how you know. 3. The Krebs cycle makes several high energy molecules, but which one is made the most and what is it used for? 4. The electron transport chain, moves electrons from one molecule to another. What happens to the energy of the electrons as they move “down the chain?” 5. What happens to protons (hydrogen ions) as a result of a functioning electron transport chain? 6. Why do you need a high concentration of protons (hydrogen ions) on one side of the membrane? Why can that help you make ATP? 7. Career connection: There are a significant number of people studying mitochondrial diseases. These are all caused by some problem in the mitochondria where the electron transport chain helps produce ATP. Research one mitochondrial disease and determine what part of the electron transport chain is damaged. SAM HOMEWORK QUESTIONS Cellular Respiration - With Suggested Answers for Teachers Use the following image showing one step in glycolysis to answer questions 1-2: 1. In the chemical reaction above, what is the name of the enzyme? phosphofructokinase 2. Is this a step in glycolysis that makes ATP or uses it up? Explain how you know. ATP is being used up here. The molecules on the left side of a reaction arrow are the reactants, the ones being used up. 3. The Krebs cycle makes several high energy molecules, but which one is made the most and what is it used for? NADH is the most common high energy molecule, and it is used in the Electron Transport Chain to pump protons across the membrane. 4. The electron transport chain, moves electrons from one molecule to another. What happens to the energy of the electrons as they move “down the chain?” The energy of the electrons decreases. This “lost” energy is used to pump protons across the membrane causing an increase of energy due to the concentration difference that occurs. 5. What happens to protons (hydrogen ions) as a result of a functioning electron transport chain? Protons are pumped from one side of the membrane to the other. 6. Why do you need a high concentration of protons (hydrogen ions) on one side of the membrane? Why can that help you make ATP? The high concentration means a high energy build up. That higher energy can be used along with ATP synthase to make ATP a high energy chemical we need to live. 7. Career connection: Most mitochondrial disorders are genetic in nature and result from impairment of the protein complexes that take part in the electron transport chain, or are involved in shuttling molecules into and out of the mitochondria. One example is Leber’s hereditary optic atrophy, in which there are problems with the genes that make the proteins for complex I in the electron transport chain.