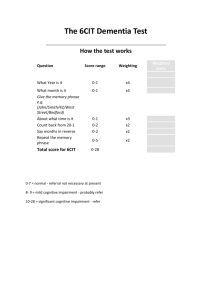
Proposed response criteria for neurocognitive impairment in
advertisement

Lupus (2007) 16, 418–425 http://lup.sagepub.com PAPER Proposed response criteria for neurocognitive impairment in systemic lupus erythematosus clinical trials Ad Hoc Committee on Lupus Response Criteria: Cognition Sub-committee Members of the Ad Hoc Committee were J Mikdashi* (Co-Chair), JM Esdaile (Co-Chair), GS Alarcón (Co-Chair), L Crofford, BJ Fessler, L Shanberg, H Brunner, V Gall, JR Kalden, MD Lockshin, MH Liang, N Roberts Jr and M Schneider The objective of this study was to identify reliable and valid instruments to measure cognitive impairment in systemic lupus erythematosus (SLE), and to define minimally important change of cognitive impairment in SLE for clinical trials. Neurocognitive measures used in randomized clinical trials in SLE were reviewed, and response criteria were developed using consensus expert opinion. The definition of cognitive impairment in the ACR nomenclature for neuropsychiatric lupus syndrome was adopted. Cognitive impairment is a deficit of 2.0 or more standard deviations (SD) below the mean, compared to normative data, in the key domains of attention, memory and psychomotor speed. Cognitive decline is defined as a deficit of 1.5–1.9 SD below the mean. Focal decline is defined if impairment exists in one or more measures within one domain, and multifocal decline if impairment exists on measures spanning two or more domains. The combination of ACR neuropsychological battery and the Cognitive Symptoms Inventory (CSI) is recommended to quantitate cognitive function. A clinically important response is defined as an improvement of ⱖ1.0 SD with an effect size of 1.0 in the key domains of the ACR neuropsychological testing, and an improvement of ⱖ1.0 SD with an effect size of 1.0 in functional performance of the CSI. Lupus (2007) 16, 418–425 Key words: clinical trials; cognitive function; cognitive impairment; neuropsychological testing; response criteria Introduction The American College of Rheumatology has begun initiatives to facilitate clinical trials in systemic lupus erythematosus (SLE) and to standardize the endpoints used. Response criteria for six measures of overall disease activity were put forth with the intention that they be used with measures for principal manifestations targeted in the clinical setting.1 A priori response criteria for proliferative and membranous renal disease in SLE has been published,2 and this paper describes the work in cognitive impairment. Patients with SLE often complain of cognitive difficulties that are not specific to one brain region or cognitive domain, and in the majority of patients are subclinical.3–8 Many of these cognitive difficulties are associated with metabolic disturbances, medications or *Correspondence: Jamal A Mikdashi, University of Maryland School of Medicine, Division of Rheumatology and Clinical Immunology, 10 S Pine Street, Suite 834, Baltimore, MD 21201, USA. E-mail: jmikdash@ umaryland.edu Received 31 October 2006; accepted 13 March 2007 © 2007 SAGE Publications prior learning disabilities and psychiatric syndromes (Table 1). In the individual patient it is often difficult to determine whether cognitive impairment is related to the disease, its complications, its treatments, or to co-morbid conditions. The ACR Nomenclature and Case Definitions for Neuropsychiatric Lupus Syndromes9 defines cognitive impairment as a decline from a higher former level of functioning in one or more of the following domains; simple or complex attention, memory (eg, learning and recall), visual-spatial processing, language (eg, verbal fluency), reasoning and problem solving, psychomotor speed, and executive functions (eg, planning, organizing and sequencing). The definition for cognitive impairment in SLE requires documentation by a neurocognitive battery.10,11 The ACR neurocognitive short battery (Table 2) was suggested to assess cognitive deficits in SLE and in assessing changes over time. It is agreed upon that a validated, brief screening measure could be used to identify patients with SLE who should be referred for formal neurocognitive evaluation. The Cognitive Symptoms Inventory (CSI)12 provides a profile of self-perceived cognitive function in 10.1177/0961203307079044 Proposed response criteria for neurocognitive impairment Ad Hoc Committee 419 Table 1 Factors that influence cognitive function Conditions Causes Cerebral pathology Structural CNS damage (eg, MRI*) Primary neurological illnesses History of learning disability History of head injury Epileptic seizures Psychosis Mania Depression Anxiety Glucocorticoids, narcotic analgesics Alternative medications (eg, herbs) Illicit drug use SLE activity Comorbid illnesses aPL** status Uremia Diabetes Others ⫹⫹ Sleep deprivation Fatigue Acute or chronic pain Age/development Sociocultural factors Psychiatric disorders Medications Toxic disorders Medical illnesses Metabolic disturbances Physiologic conditions Environmental factors *MRI ⫽magnetic resonance imaging **aPL ⫽anti-phospholipid antibodies ⫹⫹⫽electrolyte disturbances or hepatic failure. Table 2 Cognitive tests of the proposed one-hour repeatable neuropsychological battery NART (to estimate IQ)** Digit symbol substitution test Trail making test (part A & B) Stroop color and word test California verbal learning test Rey Osterrieth complex figure test (with delayed recall) WAIS III letter numbering sequencing** Controlled oral word association test Animal naming Finger tapping *NART ⫽ National Adult Reading Test, IQ ⫽ Intelligence Quotient, WAIS ⫽Wechsler Adult Intelligence Scale. **Not appropriate for use in children and adolescents. rheumatic diseases and assesses self-reported functional competency in commonly performed activities of daily living that require cognition. This inventory addresses functional capabilities that are strongly associated with other measures of cognitive impairment in SLE.4 Material and methods The ACR SLE Response Criteria Committee consists of clinicians, stakeholders and trial methodologists from the ACR, the Systemic Lupus International Collaborating Clinics (SLICC) group, the European League Against Rheumatism (EULAR), the Pan American League against Rheumatism (PANLAR), the International League Against Rheumatism (ILAR), the Food and Drug Administration (FDA) and the Outcome Measures in Rheumatology Clinical Trials (OMERACT) group. No industry funding was accepted. All participants signed Conflict of Interest statements and attested that they met the standards of ethical conduct of the ACR. Outcome measures used in cohort studies and randomized controlled clinical trials in SLE were reviewed. (JAM) using MEDLINE search of the English literature published from January1986 to June 2006; key words search included SLE, cognition, neuropsychological assessment, and outcome measures. The committee viewed the studies, assessed their quality (accurate and appropriate outcomes, clear case-control definitions, blinding, reliable assessment of disease state, appropriate statistical tests used),13 and the psychometric properties (validity, reproducibility, sensitivity) of cognitive measures. In addition, subscales of the SLEDAI, SLEDAI 2K, SELENA–SLEDAI, SLAM, SLAM-R, ECLAIM and RIFLE were inspected for their sensitivity for measuring cognition. The SLE Response Criteria Committee has recognized that the choice of endpoints and the definition of a meaningful change have to be, to some degree, arbitrary and based on consensus expert opinion. A nominal group exercise was conducted to generate a list of mutually exclusive, collectively exhaustive covariates that should be reported in studies needed to interpret the results of neurocognition. A formal vote was taken to determine those that were felt to represent minimum essential neurocognitive disease endpoints. A second exercise was done to establish a consensus for a minimally important change of the endpoints. The committee did this without fully acknowledging that data was lacking to rationalize this choice of biological importance and that this was for the purpose of standardization. The final recommendations were sent for review by invited experts in adult and pediatric rheumatology, neurology, neuropsychology and clinical epidemiology (see also acknowledgements). Our recommendations were not reviewed by ACR. Results A diversity of cognitive abnormalities has been reported in patients with SLE. The ACR has defined cognitive impairment as a decline in one or more of the following domains: simple attention, complex attention, memory, visual spatial processing, language, reasoning/problem solving, psychomotor speed and executive function.9 The findings of studies of cognitive impairment in SLE have varied with no consistent pattern of cognitive impairment established. Lupus Proposed response criteria for neurocognitive impairment Ad Hoc Committee 420 The systematic review of relevant literature identified 25 controlled studies out of 142 based on their design and the quality of their execution.14–39 Cognitive impairment was defined in most of these studies using criteria designed to be analogous to inferential testing of a two-tailed hypothesis at a significant level of 0.05. For most of these studies, cognitive impairment was said to be present if the subject’s scores were more than two standard deviations (SD) below the estimated population mean; this constituted performance for the bottom 2.5% of the general population. If the population data were not normally distributed, percentiles were calculated so that those in the lowest 2% were considered abnormal. For the 25 studies, cognitive impairment was reported in 27–61% of SLE patients, the variation probably due to differences in patient selection and in case definitions. Mean test scores were significantly lower in the domains of attention (20 studies), visual memory (16 studies), verbal memory (15 studies) and psychomotor speed (13 studies) for SLE patients as compared to controls. No significant abnormalities were, noted on measures of language, visual spatial and concept formation for most of the studies. When SLE patients who had previously experienced neuropsychiatric SLE were compared to those who have never had such involvement, severe impairment was observed among the first group. The most severe levels of impairment were in the domains of attention (16 studies), memory (12 studies), and verbal fluency (nine studies). Neurocognitive assessment Neurocognitive assessment analyses cognitive and behavioural disturbances resulting from abnormal brain development, head trauma, or other disorders.11 Techniques to measure cognitive performances or its impact were reviewed by the committee. Table 3 Cognitive symptoms inventory questionnaire* Dial a telephone Organize preparing meals at home Recognize people you know Learn new things See different colors only as black and white Remember details of your recent experiences Remember important experience in your past Shop for food or things you need without a list Manage moneymaking change, pay bills Remember to take your medications, as you should Remember details at home or at work Stay awake during a movie or TV show Concentrate on a task you need to do Concentrate on more than one task at a time Find the correct word during conversations Remember why you came into a room Remember where you put things such as keys and glasses Remember what you ate or wore two days ago Find your way while driving Keep track of things to do without a list The 21 self-report cognitive symptoms inventory questionnaire concerns possible problems in every day activities over last month. The following is a score system to each question asked, ranging between never a problem (1), a problem some of the time (2), a problem most of time (3), a problem all of time/unable to do (4), and not applicable (0). *Not appropriate for use in children and adolescents. qualitative information. The interpretation of test results and the identification of cognitive impairment are based on normative data appropriate for age, education, gender and ethnic group.4,8,11 Many neurocognitive test batteries are available and often require more than one hour to administer. The battery (Table 2) of the ACR attempts to standardize and make testing more practical includes components from other batteries that have been used in lupus15–39 and non-lupus patients44–48 to screen for cognitive impairment, and follow patients longitudinally. The ACR battery is valid and reliable,19 does not have significant motor demands and takes about one hour to perform. Formal functional assessment. This assesses the impact Neurocognitive tests. Several screening tests identify cognitively impaired patients. These include the MiniMental State,40 the Cognitive Capacity Screening examination41 and the Neurobehavioral Cognitive Status examination.42 These tests have substantial false negative rates, do not cover all domains of cognitive functioning, fail to detect early or milder forms of cognitive impairment, are relatively insensitive to detect changes overtime, and are influenced by the subject’s sociodemographic features. Formal neurocognitive testing assesses cognitive task performance and provides an objective, comprehensive and non-invasive evaluation of cerebral functioning.43 These tests are administered and scored in a standardized fashion and yield quantitative and Lupus of cognitive performance on the individuals. Such tests measure the level of day-to-day functioning indicative of cognition. The integration of cognitive measures and functional assessment of social and occupational domains are important evidence required for the diagnosis of cognitive impairment. The CSI is a selfreported instrument and was felt best used as a screen for difficulties in daily activities involving intermediate memory, concentration, attention, and executive function12 to determine the level of assistance the patient may need, and can be used to monitor changes that occur over time (Table 3). The scales of instruments measuring overall disease activity reviewed by the committee included SLEDAI and SLEDAI-2K the Systemic Lupus Activity Measure Proposed response criteria for neurocognitive impairment Ad Hoc Committee 421 (SLAM, SLAM/R), the British Isles Lupus Assessment Group (BILAG), the European Consensus Lupus Activity Measurement (ECLAM), and the Response Index For Lupus Erythematosus (RIFLE). The committee felt coverage of cognitive function by these scales was not sufficiently sensitive. Most of prior studies have found no correlation between cognitive impairment and disease activity as measured with any of the instruments mentioned.5,23,30–33,39,49 Similarly, most studies found no correlation between SLICC/ACR damage index (SDI) scores and cognitive impairment.4,16,24,25,50 Neuroimaging technique [computed tomography, conventional magnetic resonance, magnetic resonance spectroscopy, magnetization transfer imaging, single photon emission computed tomography (SPECT), positron emission tomography (PET), cerebral blood flow measurements]51–60 and serological markers of cognitive function or brain damage, including antiphospholipid antibodies,61–65 anti–N-methyl-D-aspartate (NMDA) antibodies14,66 and other biologic markers,14,66–71 were considered but the data and the experience with them were insufficient to judge their sensitivity to change and their prognostic value. Moreover an indeterminate number of SLE patients with and without cognitive impairment have these markers. Assessment of neurocognitive functioning in children with SLE Neurocognitive involvement in pediatric SLE is an important problem.72,73 In a small cohort study children with SLE were found to have more problems acquiring new cognitive skills when compared to their peers.74 The ACR definition of cognitive impairment has not been validated in children. The assessment of neurocognitive functioning in children entails unique challenges that complicate the already difficult problem of determining the presence of clinically significant cognitive impairment due to SLE. Furthermore, both testing techniques and aspects of normal cognitive function, change dramatically with age and development, making serial testing more difficult to interpret.75 Children tend to have shorter attention spans and be more prone to fatigue than their adult counterparts, negatively influencing lengthy testing batteries and threatening the validity of test results.76 Moreover, standard definitions for test interpretation in children must consider age related normative data. Accordingly, available assessment instruments may not be applicable across the entire age range of children diagnosed with SLE. Despite the apparently high prevalence of neurocognitive involvement in pediatric SLE,72 there are no large published studies addressing the usefulness of available neurocognitive assessment instruments in these populations. However, initial validation studies of the pediatric version of the ANAM suggest this tool may be useful for assessing cognitive functioning of children as young as eight years of age in a clinical setting.77 Finally, preliminary evidence suggests that self report of subjective cognitive symptoms in children is unreliable to screen for neurocognitive dysfunction, in sharp contrast to what has been shown in adults.72 Recommendations of the committee The committee uses the ACR definition of cognitive impairment. Cognitive impairment is said to present if the score is 2.0 or more SD below the mean in key domains of attention, memory, and psychomotor speed, compared to normative data using the ACR neurocognitive battery, whereas, cognitive decline is defined as a score 1.5–1.9 SD below the mean. Cognitive impairment is considered focal if one or more measures within one domain are affected and multifocal when measures spanning two or more domains are affected. The committee recommends the ACR neurocognitive battery and the CSI be used in studies directed at therapeutic or rehabilitative strategies. These instruments may be used to screen for cognitive impairment in SLE and to follow patients longitudinally. However, it should be noted that we could not identify any specific study of whether longitudinal monitoring is useful prognostically or therapeutically. The current recommendations are only appropriate for the assessment of neurocognitive impairment in adults with SLE. Clinically important response (Table 4) The ACR neurocognitive battery. A clinically important response is defined if improvement of ⱖ1.0 SD with an effect size of ⱖ1.0 in a key domain or restoration of normal performance in the battery. The Cognitive Symptom Inventory. A clinically important response is defined if an improvement in ⱖ1.0 SD in functional response with an effect size of ⱖ1.0. Table 4 Clinically important response A) ACR neuropsychological battery A change of ⱖ1.0 SD with an effect size of ⱖ1.0, better or worse in key domain B) Cognitive Symptom Inventory A change of ⱖ1.0 SD with an effect size of ⱖ1.0 ACR ⫽American College of Rheumatology. SD ⫽standard deviation. Lupus Proposed response criteria for neurocognitive impairment Ad Hoc Committee 422 Discussion The conduct of clinical trials of interventions where neurocognitive SLE is the therapeutic target is challenged by the heterogeneity of SLE patients with cognitive impairment, the various patterns of cognitive abnormalities observed, and the lack of surrogate markers. Defining standardized clinical response criteria for cognitive impairment in SLE allows the testing of new therapeutic approaches and permits the pooling of data and comparison of results across SLE populations. These tools can also be used to measure side effects of medications, sleep disturbance, fatigue and other factors which are known to affect cognitive performance. The committee has addressed various neurocognitive tools including the computer-administered test battery (automated neuropsychological assessment metrics — ANAM)8,14,50 and the 42-item questionnaire adapted by Denburg et al.78 Beyond ease of administration and data collection, the computer-based assessment offers other benefits over paper-and-pencil testing in the form of millisecond timing accuracy, reliable and randomized presentation of stimuli over multiple trials and repeat administrations, and unobtrusive measurement of cognitive skills and response times during the assessment process. Therefore, this instrument may be particularly useful for longitudinal assessment of change. However, the use of the automated neurocognitive testing is limited with respect to predicting the nature and the extent of cognitive impairment, and lacking the degree of sophistication necessary to produce the same results as a human assessor’s professional judgment that combines test data with other pertinent clinical variables.79 Thus, the advantages and disadvantages of computerized methods must be considered, in anticipation of the development of psychometric data based on comparisons with long-standing empirically sound test measures, and the use of computerized adaptive testing tailored towards SLE patients. Assessment of neurocognitive functioning in children and adolescents with SLE requires consideration of the developmental stage and age of the child. As a result, standardized testing batteries for children may require additional measures and more time to complete than those for adult patients. An ongoing effort by the Childhood Arthritis and Rheumatology Research Alliance (CARRA) will lead to consensus on a suitable standardized pediatric SLE neurocognitive battery; however, the evidence required for such recommendations does not exist at this time. Nevertheless, several of the tests recommended for adults in these guidelines are known to be inappropriate for children and adolescents, including the CSI, National Adult Reading Lupus Test (NART), and Wechsler Adult Intelligence Scale (WAIS). The use of the 42-item questionnaire evaluates nonspecific neurological soft signs that may influence cognitive performance and correlate with a generalized cognitive deficit rather than to any specific cognitive function. Though this questionnaire may be useful occasionally as an indicator of global cerebral dysfunction of various etiologies, it is not used to confirm impression of global impairment or to monitor changes in cognitive functions. A fundamental difficulty with cognitive testing is the control of independent variables, which sometimes can be addressed by grouping patients according to the clinical manifestations of neuropsychiatric SLE, socio-demographic status or adjusting for co-morbidities. However, it is important to recognize that individuals grouped in this fashion might not necessarily exhibit similar patterns of cognitive impairment.80,81 A critical component in the diagnosis of mild cognitive impairment is to determine whether every day functioning is impacted by the subject’s cognitive deficits. To optimize efficiency in neurocognitive testing, functional assessment may contribute to the profile of cognitive impairment. Our Committee recommends the use of the CSI, as indicative of cognitive function, for the screening patients suspected of having cognitive impairment in the research and clinical settings. As noted by Alarcón et al.4 and others,82 the CSI appears not to be affected by the patient’s sociodemographic background. However, it is necessary to establish the psychometric properties of the CSI; validation studies must examine the relationship between the patients’ CSI scores and their performance on the ACR neurocognitive battery. The goal of most studies of cognitive function in patients groups is to demonstrate that a decline in function has reached a level such that it provides a statistically significant difference between the performance of the patient and control groups. Determining what constitutes a real change between tests or following an intervention relies heavily on the reliability and validity of the psychometric tests, but most importantly on what is regarded as clinically significant or meaningful change. However, in clinical trials, it is not always evident that statistically significant differences in cognitive function are clinically important. Although much work remains, this is the first attempt to define response criteria for clinical trials for cognitive impairment in SLE. The ACR neurocognitive battery and the CSI assess all major areas of cognitive function and are suitable for individual patients. Because of heterogeneity and comorbidities, subanalysis should be based on segregating patients into Proposed response criteria for neurocognitive impairment Ad Hoc Committee 423 groups emphasizing the complexity of neurocognitive complications in SLE. Further research should be devoted to validate these measures in randomized clinical trials and longitudinal observational studies, and to identify biological markers and, possibly, neuroimaging techniques in the measurement of response. Acknowledgements We gratefully acknowledge the logistical and staff support of Ms Amy Miller, the secretarial contribution of Ms Angela Strickland and the invaluable input from Dr Robin Brey, Dr Melanie Harrison, Dr Lynn Grattan, Dr Deborah M Levy, Dr Kelly Anthony and Dr Marisa Klein-Gitelman. Supported by grants from the American College of Rheumatology, a Kirkland Scholar Award, the SLE Foundation of New York, The Lupus Erythematodes Selbsthilfegemeinschaft e. V. Germany, NIH Grant number AR47782, R13 AR47584-01, Robert B. Brigham Arthritis and Musculoskeletal Diseases Clinical Research Center (Harvard University), the Heinrich-Heine-University in Düsseldorf, the Arthritis Research Centre of Canada (University of British Columbia), the Massachusetts Veterans Epidemiology Research and Information Center, and the Center for Advanced Methodological Support for Innovative SLE Clinical Trials (ASSIST). References 1 American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Response Criteria. The American College of Rheumatology response criteria for systemic lupus erythematosus clinical trials: Measures of overall disease activity. Arthritis & Rheumatism 2004; 50: 3418–3426. 2 Liang M, Schur P, Fortin P et al. The American college of rheumatology response criteria for proliferative and membranous renal disease in systemic lupus erythematosus clinical trials. Arthritis & Rheumatism 2006; 54: 421–432. 3 Kremer JN, Rynes RI, Bartholomew LE et al. Non-organic nonpsychotic psychopathology (NONPP) in patients with systemic lupus erythematosus. Seminars Arthritis Rheum 1981; 11: 182–189. 4 Alarcón GS, Cianfrini L, Bradley LA et al. Systemic lupus erythematosus in three ethnic groups: Measuring cognitive impairment with the cognitive symptoms inventory. Arthritis Rheum 2002; 47: 310–319. 5 Hanly JG, Fisk JD, Sherwood G, Eastwood B. Clinical course of cognitive dysfunction in systemic lupus erythematosus. J Rheumatol 1994; 10: 1825–1831. 6 Hanly JG, Cassell K, Fisk JD. Cognitive function in systemic lupus erythematosus: result of 5-year prospective study. Arthritis Rheum 1997; 40: 1542–1543. 7 Denburg SD, Carbotte RM, Denburg JA. Cognition and mood in systemic lupus erythematosus. Evaluation and pathogenesis. Ann N Y Acad Sci 1997; 823: 44–59. 8 Brey RL, Holliday SL, Saklad AR et al. Neuropsychiatric syndromes in lupus: prevalence using standardized definitions. Neurology 2002; 58: 1214–1220. 9 Ad hoc Committee. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum 1999; 42: 599–608. 10 Kaplan H, Sadock B. Comprehensive textbook of psychiatry, 7th edition. Williams & Wilkins, 2000. 11 Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment, 4th edition. Oxford University Press, 2004. 12 Pincus T, Swearingen C, Callahan LF. A self-report cognitive symptoms inventory to assess patients with rheumatic diseases: Results in eosinophilia-myalgia syndrome (EMS), fibromyalgia, rheumatoid arthritis (RA), and other rheumatic diseases. Arthritis Rheum 1996; 39: S261. 13 Jadad AR, Cook DJ, Jones A et al. Methodology and reports of systematic reviews and meta-analyses: a comparison of Cochrane reviews with articles published in paper-based journals. JAMA 1998; 280: 278–280. 14 Takada K, Jacobs G, Lapteva L et al. Use of computerized assessment to predict neuropsychological functioning and emotional distress in patients with systemic lupus erythematosus. Arthritis & Rheum 2006; 55: 434–441. 15 Glanz BI, Schur P, Lew R, Khoshbin S. Lateralized cognitive dysfunction in patients with systemic lupus erythematosus. Lupus 2005; 14: 896–902. 16 Doninger NA, Fink JW, Utset TO. Neuropsychologic functioning and health status in systemic lupus erythematosus: does ethnicity matter? J Clin Rheumatol 2005; 11: 250–256. 17 Emori A, Matsushima E, Aihara O et al. Cognitive dysfunction in systemic lupus erythematosus. Psychiatry Clin Neurosci 2005; 59: 584–589. 18 Shucard JL, Parrish J, Shucard DW, McCabe DC, Benedict RH, Ambrus J Jr. Working memory and processing speed deficits in systemic lupus erythematosus as measured by the paced auditory serial addition test. J Int Neuropsychol Soc 2004; 10: 35–45. 19 Kozora E, Ellison MC, West S. Reliability and validity of the proposed American College of Rheumatology neuropsychological battery for systemic lupus erythematosus. Arthritis Rheum 2004; 51: 810–818. 20 Loukkola J, Laine M, Ainiala H et al. Cognitive impairment in systemic lupus erythematosus and neuropsychiatric systemic lupus erythematosus: a population-based neuropsychological study. J Clin Exp Neuropsychol 2003; 25: 145–151. 21 Leritz E, Brandt J, Minor M, Reis-Jensen F, Petri M. Neuropsychological functioning and its relationship to antiphospholipid antibodies in patients with systemic lupus erythematosus. J Clin Exp Neuropsychol 2002; 24: 527–533. 22 Kozora E, Laudenslager M, Lemieux A, West SG. Inflammatory and hormonal measures predict neuropsychological functioning in systemic lupus erythematosus and rheumatoid arthritis patients. J Int Neuropsychol Soc 2001; 7: 745–754. 23Monastero R, Bettini P, Del Zotto E et al. Prevalence and pattern of cognitive impairment in systemic lupus erythematosus patients with and without overt neuropsychiatric manifestations. J Neurol Sci 2001; 184: 33–39. 24 Ainiala H, Loukkola J, Peltola J, Korpela M, Heitaharju A. The prevalence of neuropsychiatric syndromes in systemic lupus erythematosus. Neurology 2001; 57: 276–283. 25 Gladman DD, Urowitz MB, Slonim D et al. Evaluation of predictive factors for neurocognitive dysfunction in patients with inactive systemic lupus erythematosus. J Rheumatol 2000; 27: 2367–2371. 26 Skeel Rl, Johnstone B, Yangco DT, Walker SE, Komatireddy GR. Neuropsychological deficit profiles in systemic lupus erythematosus. Appl Neuropsychol 2000; 7: 96–101. 27 Carlomagno S, Migliaresi S, Ambrosone L, Sannino M, Sangnes G, Di Lorio G. Cognitive impairment in systemic lupus erythematosus: a follow up study. J Neurol 2000; 247: 273–279. 28 Sabbadini MG, Manfredi AA, Bozzolo E et al. Central nervous system involvement in systemic lupus erythematosus patients without overt neuropsychiatric manifestations. Lupus 1999; 8: 11–19. 29 Breitbach S, Alexander R, Daltry L et al. Determinants of cognitive performance in systemic lupus erythematosus. J Clin Exp Neuropsychol 1998; 20: 157–166. 30 Glanz BI, Slonim D, Urowitz MB, Gladman DD, Gough J, MacKinnon A. Pattern of neuropsychologic dysfunction in inactive systemic lupus erythematosus. Neuropsychiatry Neuropsychol Behav Neurol 1997; 10: 232–238. Lupus Proposed response criteria for neurocognitive impairment Ad Hoc Committee 424 31 Kozora E, Thompson LL, West SG, Kotzin BL. Analysis of cognitive and psychological deficits in systemic lupus erythematosus patients without overt central nervous system disease. Arthritis Rheum 1996; 39: 2035–2045. 32 Carbotte RM, Denburg SD, Denburg JA. Cognitive dysfunction in systemic lupus erythematosus is independent of active disease. J Rheumatol 1995; 22: 863–867. 33 Hanly J, Fisk J, Sherwood G, Jones E, Jones J, Eastwood B. Cognitive impairment in patients with systemic lupus erythematosus. J Rheumatol 1992; 19: 562–567. 34 Hay EM, Black D, Huddy A et al. Psychiatric disorder and cognitive impairment in SLE. Arthritis Rheum 1992; 35: 411–416. 35 Ferstl R, Niemann T, Biehl G, Hinrichsen H, Kirch W. Neuropsychological impairment in auto-immune disease. Eur J Clin Invest 1992; 22(Suppl 1): 16–20. 36 Ginsburg KS, Wright EA, Larson MG et al. A controlled study of the prevalence of cognitive dysfunction in randomly selected patients with systemic lupus erythematosus. Arthritis Rheum 1992; 35: 776–782. 37 Wekking E, Nossent J, van Dam A, Swaak A. Cognitive and emotional disturbances in systemic lupus erythematosus. Psychother Psychosom 1991; 55: 126–131. 38 Denburg SD, Carbotte RM, Denburg JA. Cognitive impairment in systemic lupus erythematosus: a neuropsychological study of individual and group deficits. J Clin Exp Neuropsychol 1987; 9: 323–339. 39 Carbotte RM, Denburg SD, Denburg JA. Prevalence of cognitive impairment in systemic lupus erythematosus. J Nerv Ment Dis 1986; 174: 357–364. 40 Folstein MF, Folstein SE, McHugh PR. Mini-mental state. J Psychiatr Res 1975; 12: 189–198. 41 Nelson A, Fogel BS, Faust D. Bedside cognitive screening instruments: a critical assessment. J Nerv Ment Dis 1986; 174: 73–83. 42 Keirnan RJ, Mueller J, Langstone JW, van Dyke C. The neurobehavioral cognitive status examination. Ann Intern Med 1987; 107: 481–485. 43 American Psychiatric Association. Diagnostic and statistical manual of mental disorders. TR Plus, 4th edition. American Psychiatric Association, 2000. 44 Smith RC, Infante M, Singh A, Khandat A. The effects of olanzapine on neurocognitive functioning in medication refractory schizophrenia. Int J Neuropsychpharmacol 2001; 4: 239–250. 45 Cohen RA, Boland R, Paul R et al. Neurocognitive performance enhanced by highly active retroviral therapy in HIV infected women. AIDS 2001; 15: 341–345. 46 Grigore AM, Grott HP, Mathew JP et al. The rewarming rate and increased peak temperature alter neurocognitive outcome after cardiac surgery. Anesth Analg 2002; 94: 4–10. 47 Barak Y, Achiron A. Effect of interferon-beta-1b on cognitive functions in multiple sclerosis. Eur Neurology 2002; 47: 11–14. 48 Winblad B, Engedal K, Soininen H et al. A one- year randomized placebo controlled study of donepezil in patients with mild to moderate AD. J Neurology 2001; 57: 489–495. 49 Sweet JJ, Doninger NA, Zee PC, Wagner LI. Factors influencing cognitive function, sleep, and quality of life in individuals with systemic lupus erythematosus: a review of the literature. Clin Neuropsychol 2004; 18: 132–147. 50 Holliday SL, Navarrete MG, Hermosillo-Romo D et al. Validating a computerized neuropsychological test battery for mixed ethnic lupus patients. Lupus 2003; 12: 697–703. 51 Sibbitt WL Jr, Sibbitt RR, Brooks WM. Neuroimaging in neuropsychiatric lupus. Arthritis Rheum 1999, 42: 2026–2038. 52 Kozora E, Arciniegas D, Filley C et al. Cognition, MRS neurometabolites, and MRI volumetrics in non-neuropsychiatric systemic lupus erythematosus: preliminary data. Cogn Behav Neurol 2005; 18: 159–162. 53 Sailer M, Burchert W, Ehrenheim C et al. Positron emission tomography and magnetic resonance imaging for cerebral involvement in patients with systemic lupus erythematosus. J Neurol 1997; 244: 186–193. 54 Komatsu N, Kodama K, Yamanouchi N et al. Decreased regional cerebral metabolic rate for glucose in systemic lupus erythematosus patients with psychiatric symptoms. Eur Neurol 1999; 42: 41–48. Lupus 55 Kozora E, West SG, Kotzin BL, Julian L, Porter S, Bigler E. Magnetic resonance imaging abnormalities and cognitive deficits in systemic lupus erythematosus patients without overt central nervous system disease. Arthritis Rheum 1998; 41: 41–47. 56Waterloo K, Omdal R, Jacobsen EA et al. Cerebral computed tomography and electroencephalography compared with neuropsychological findings in systemic lupus erythematosus. J Neurology 1999; 246: 706–711. 57 Waterloo K, Omdal R, Sjoholm H et al. Neuropsychological dysfunction in systemic lupus erythematosus is not associated with changes in cerebral blood flow. J Neurol 2001; 248: 595–602. 58 Bosma GP, Rood MJ, Huizinga TW et al. Detection of cerebral involvement in patients with active neuropsychiatric systemic lupus erythematosus by the use of volumetric magnetization transfer imaging. Arthritis Rheum 2000; 43: 2428–2436. 59 Govoni M, Castellino G, Padovan M, Borrelli M, Trotta F. Recent advances and future perspective in neuroimaging in neuropsychiatric systemic lupus erythematosus. Lupus 2004; 13: 149–158. 60 Steens SC, Admiraal-Behloul F, Bosma GP et al. Selective gray matter damage in neuropsychiatric lupus. Arthritis Rheum 2004; 50: 2877–2881. 61 Hanly JG, Hong C, Smith S, Fisk JD. A prospective analysis of cognitive function and anticardiolipin antibodies in systemic lupus erythematosus. Arthritis Rheum 1999; 42: 728–734. 62 Denburg SD, Carbotte RM, Ginsberg JS, Denburg JA. The relationship of antiphospholipid antibodies to cognitive function in patients with systemic lupus erythematosus. J Int Neuropsychol Soc 1997; 3: 377–386. 63 Leritz E, Brandt J, Minor M, Reis-Jensen F, Petri M. Neuropsychological functioning and its relationship to antiphospholipid antibodies in patients with systemic lupus erythematosus. J Clin Exp Neuropsychol 2002; 24: 527–533. 64 Afeltra A, Garzia P, Mitterhofer AP et al. Neuropsychiatric lupus syndromes: relationship with antiphospholipid antibodies. Neurology 2003; 61: 108–110. 65 Menon S, Jameson-Shortall E, Newman SP, Hall-Craggs MR, Chinn R, Isenberg DA. A longitudinal study of anticardiolipin antibody levels and cognitive functioning in systemic lupus erythematosus. Arthritis Rheum 1999; 42: 735–741. 66 Kowal C, DeGiorgio LA, Nakaoka T et al. Cognition and immunity; antibody impairs memory. Immunity 2004; 21: 179–188. 67 Long AA, Denburg SD, Carbotte RM, Singal DP, Denburg JA. Serum lymphocytotoxic antibodies and neurocognitive function in systemic lupus erythematosus. Ann Rheum Dis 1990; 49: 249–253. 68 Brooks WM, Jung RE, Ford CC, Grienel EJ, Sibbitt WL. Relationship between neurometabolite derangement and neurocognitive dysfunction in systemic lupus erythematosus. J Rheumatol 1999; 26: 81–85. 69 Trysberg E, Nylen K, Rosengren LE, Tarkowski A. Neuronal and astrocytic damage in systemic lupus erythematosus patients with central nervous system involvement. Arthritis Rheum 2003; 48: 2881–2887. 70 Trysberg E, Blennow K, Zachrisson O, Tarkowski A. Intrathecal levels of matrix metalloproteinases in systemic lupus erythematosus with central nervous system engagement. Arthritis Res Ther 2004; 6: 551–556. 71 Ainiala H, Hietaharju A, Dastidar P et al. Increased serum matrix metalloproteinase 9 levels in systemic lupus erythematosus patients with neuropsychiatric manifestations and brain magnetic resonance imaging abnormalities. Arthritis Rheum 2004; 50: 858–865. 72 Sibbitt W, Brandt J, Johnson C, Maldonado M et al. The incidence and prevalence of neuropsychiatric syndromes in pediatric onset systemic lupus erythematosus. J Rheumatol 2002; 29: 1536–1542. 73 Steinlin M, Blaser S, Gilday D, Eddy A et al. Neurologic manifestations of pediatric systemic lupus erythematosus. Pediatric Neurology 1995; 13: 191–197. 74 Klein-Gitelman MS, Zelko F, Kress A et al. Comparison of neurocognitive function in children with pediatric systemic lupus erythematosus (pSLE) and their peers – second year follow up. Arthritis Rheum 2002; 46: S216. 75 Sattler JM. Issues related to the measurement and change of intelligence. Assessment of children: cognitive applications, 4th edition. Sattler J.M., 2001: 160–182. Proposed response criteria for neurocognitive impairment Ad Hoc Committee 425 76 Glutting JJ, Oakland T, McDermott PA. Observing child behavior during testing: Constructs, validity, and situational generality. Journal of School Psychology 1989; 27: 155–164. 77 Ruth N, German A, Graham TB, Passo M, Ris D, Brunner HI. Diagnosis of childhood-onset lupus neurocognitive impairment in a clinical setting: usefulness of computer based testing and self-report. Arthritis Rheum 2006; 54: S688. 78 Denburg SD, Stewart KE, Hart LE, Denburg JA. How “soft” are soft neurological signs? The relationship of subjective neuropsychiatric complaints to cognitive function in systemic lupus erythematosus. J Rheumatol 2003; 30: 1006–1010. 79 Butcher JN, Perry J, Hahn J. Computers in clinical assessment: historical developments, present status, and future challenges. J Clin Psychol 2004; 60: 331–345. 80 Ojemann G, Ojemann J, Lettich E. Cortical language localizations in left, dominant hemisphere: an electrical stimulation mapping experiment in 117 patients. J Neurosurg 1989; 71: 316–326. 81 Marshall JC. The description and interpretation of aphasic language disorder. Neuropsychologia 1986; 24: 5–23. 82 Hanly J, Fisk J, McCurdy G, Fougere L, Douglas J. Neuropsychiatric syndromes in patients with systemic lupus erythematosus and rheumatoid arthritis. J Rheumatol 2005; 32: 1459–1464. Lupus