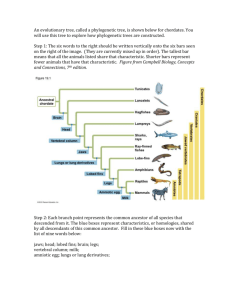
Do small mammals affect plant diversity?
advertisement

University of Münster Department of Behavioural biology Do small mammals affect plant diversity? Field studies in Namaqualand, South Africa, a biodiversity-hotspot Diploma thesis Presented by Christina Keller - April 2005 - University of Münster Department of Behavioural biology Do small mammals affect plant diversity? Field studies in Namaqualand, South Africa, a biodiversity-hotspot Diploma thesis Presented by Christina Keller - April 2005 - Contents I 1.1. Abstract...........................................................................................................1 1.2. Zusammenfassung.........................................................................................2 2. Introduction .......................................................................................................3 3. Subjects, Material and Methods.......................................................................9 3.1. Study area....................................................................................................9 3.2. Animals ......................................................................................................11 3.3. Correlation between small mammals and plants........................................14 3.3.1. Trapping............................................................................................14 3.3.2. Vegetation survey .............................................................................14 3.3.3. Soil samples .....................................................................................16 3.3.4. Altitude..............................................................................................17 3.3.5. Rainfall..............................................................................................17 3.3.6. Statistics ...........................................................................................17 3.4. Food Preference tests................................................................................18 3.5. Plant biodiversity around bush Karoo rat nests ..........................................19 3.6. Fence line...................................................................................................20 4. Results .............................................................................................................22 4.1. Correlation between small mammals and plants........................................22 4.1.1. Comparison between winter and summer.........................................22 4.1.2. Winter trapping season .....................................................................24 4.1.3. Summer trapping season..................................................................26 4.1.4. Correlation between plant cover and small mammals.......................29 4.1.5. Soil survey ........................................................................................29 4.1.6. Cluster-analyses ...............................................................................32 4.1.7. General linear model.........................................................................32 4.2. Food Preference tests................................................................................34 Contents II 4.2.1. Pilot study .........................................................................................34 4.2.1.1. Striped mouse (R. pumilio) ....................................................34 4.2.1.2. bush-Karoo rat (O. unisulcatus).............................................34 4.2.2. Second set of tests ...........................................................................35 4.2.2.1. Striped mouse (R. pumilio) ....................................................35 4.2.2.2. bush-Karoo rat (O. unisulcatus).............................................36 4.3. Plant biodiversity around bush Karoo rat nests ..........................................37 4.4. Fence line...................................................................................................38 5. Discussion .......................................................................................................39 5.1 Correlation between small mammals and plants.........................................39 5.2. Food-preference-tests................................................................................42 5.2.1. Pilot study .........................................................................................43 5.2.2. Second set of tests ...........................................................................43 5.3. Plant diversity around bush-Karoo rat nests...............................................44 5.4. Other factors that might influence plant biodiversity...................................45 5.5. Fence line...................................................................................................47 6. Conclusions.....................................................................................................50 7. References .......................................................................................................51 8. Appendix ..........................................................................................................57 9. Acknowledgements.........................................................................................66 Abstract 1 1.1. Abstract The conservation of species is one of the most important duties of our century. Basic ecological knowledge is essential in order to perform it. Conservation is particularly effective in hotspots of biodiversity, because many species can be protected here at the same time in a relative small area. One of these biodiversity hotspots is the Succulent Karoo in southern Africa, which holds an extraordinary high number of plant species. Small mammals are abundant in the Succulent Karoo and might be of crucial importance as herbivores in this ecosystem. For the first time the influence of small mammals on plant diversity was investigated in my study. It is known from earlier studies that herbivores can increase floral diversity by reducing dominant plant species and thus providing space for subdominant species, which would be outcompeted otherwise. In a correlative study I tested if this mechanism might exist in the Succulent Karoo. The plant diversity in 10 ecological different study sites in Goegap Nature Reserve was correlated with the number of small mammals living there. Additionally two rodent species (Rhabdomys pumilio and Otomys unisulcatus) were taken as example-species and tested in food-preference-tests for a preference for subdominant or dominant plant species. Additionally the influence of O. unisulcatus on the plant community surrounding their nests was also investigated. I found several positive correlations between plant diversity and the number of individuals and especially the species number of small mammals. The direct surroundings of occupied O. unisulcatus nests showed a significantly higher plant diversity than control areas, although food-preference tests revealed that O. unisulcatus prefers subdominant foodplants. In the contrary R. pumilio preferred dominant food-plants. All in all this results indicate a distinct influence of small mammals on plant diversity. The results of my study are of great importance for conservation programs in the Succulent Karoo in which small mammals should be included in the future. Zusammenfassung 2 1.2. Zusammenfassung Artenschutz ist eine der wichtigsten Aufgaben unserer Zeit. Für seine Durchführung ist ökologisches Basiswissen zwingend erforderlich. Besonders effektiv ist Artenschutz an Schwerpunkten der Artenvielfalt (Biodiversität), denn hier lassen sich viele Arten gleichzeitig und auf kleinem Raum schützen. Einer dieser Biodiversitätshotspots ist die Sukkulentenkaroo im südlichen Afrika, die sich im Besonderen durch ihre extrem artenreiche Flora auszeichnet. Kleinsäuger sind hier als Pflanzenfresser von großer Bedeutung. Erstmals wurde in dieser Studie der Einfluss von Kleinsäugern auf die Artenvielfalt der Pflanzen in der Sukkulentenkaroo untersucht. Es ist aus andern Studien bekannt, dass Pflanzenfresser einen positiven Einfluss auf die Diversität ihrer Futterpflanzen haben können, indem sie dominante Pflanzenarten reduzieren und auf diese Weise Platz für subdominante Arten schaffen, die andernfalls verdrängt würden. Ob dies in der Sukkulentenkaroo der Fall ist wurde mit einer korrelativen Studie untersucht. Die Pflanzendiversität an 10 ökologisch verschiedenen Untersuchungsgebieten im Goegap Nature Reserve wurde mit den dort lebenden Kleinsäugern in Zusammenhang gebracht. Zusätzlich wurde mit Futter-PräferenzTests exemplarisch an zwei Nagerarten (Rhabdomys pumilio, Otomys unisulcatus) getestet ob sie dominante Futterpflanzen bevorzugt fressen. Bei einer dieser Arten wurde außerdem ihr Einfluss auf die Pflanzendiversität in unmittelbarer Umgebung ihres Nestes untersucht. Es wurden mehrfach positive Korrelationen zwischen der Anzahl der Kleinsäugerindividuen und besonders der Anzahl ihrer Arten und der Pflanzendiversität gefunden. Dieser Zusammenhang war im Winter deutlicher als im Sommer. Im Vergleich zu unbewohnten Gebieten wurden in unmittelbarer Umgebung von bewohnten Otomys unisulcatus-Nestern signifikant mehr Pflanzen gefunden, obwohl Futter-Präferenz-Tests zeigten, dass diese Art subdominante Pflanzenarten bevorzugt. Rhabdomys pumilio hingegen bevorzugte dominante Futterpflanzen. Diese Ergebnisse zeigen einen deutlichen Einfluss von Kleinsäugern auf die Diversität der Pflanzen ihrer Umgebung. Der ökologische Hintergrund ist von großer Bedeutung für Artenschutzprogramme in diesem gefährdeten Gebiet, in die Kleinsäuger in Zukunft einbezogen werden sollten. Introduction 3 2. Introduction The phenomenon of biodiversity is one of the most fascinating in biology. In this study biodiversity is understood as the number of different species in a certain area without regards to endemism or abundance. When Darwin first described the process of evolution, biologists began to understand the scientific basis of diversity. In the process of adaptive radiation and adaptation to ecological niches, evolution created numerous different species of plants and animals. But these species are not distributed evenly over the planet. In 2000 Myers et al identified 25 hotspots of biodiversity. These areas were chosen for their species richness, endemism, taxonomic uniqueness, unusual ecological or evolutionary phenomena and global rarity. Myers et al. saw the identification of diversity hotspots as a tool for the improvement of conservation management. All the hotspots are facing extreme threats from human interference. As conservation budgets are insufficient given the number of species threatened with extinction it is highly important to be able to support the greatest number of species at the least cost. This is much easier after identifications of biodiversityhotspots, since all hotspots together cover only 11.8 percent of the planet’s land surface, but include no less then 44 percent of the worlds plants and 35 percent of terrestrial vertebrates. Myers included only terrestrial ecosystems. Examples of these hotspots are Madagascar, Brazil’s Atlantic forest and the Tropical Andes. Identifying biodiversity is the first step understanding is the second. Several authors established hypotheses trying to explain gradients of biodiversity. Biodiversity is for example often connected with the number of available niches and the strength of genetic drift (Ihlenfeldt 1994, Connell 1964). According to Jürgens et al. (1999) a high population turnover can decrease the competition for an ecological niche in plants. Normally competition for resources limits the sharing of the same ecological niche by several species, but if species have a short lifespan, this exposure to competition is of relative short duration. In addition there is always space for new recruits to become establish. This can lead to increased biodiversity. Another potential mechanism to create or maintain diversity is the predation hypothesis first formulated by Paine (1966). By keeping the abundance of their prey in check and thus prevent competitive exclusion, predators can maintain a Introduction 4 higher diversity of prey species than would occur in their absence (Paine 1966, 1971). Already Darwin realised in 1859 that the diversity in a meadow decreases if cutting is stopped. Paine (1966) investigated connections between predators and their prey on a reef. He found that the removal of predators (i.e. the sea star Pisaster ochraceus) led to decreased diversity of their prey, mussels (e.g. Mytilus californianus) in this case. Lubchenco (1978) noticed the same phenomenon in tidal pools. Here, the presence of snails led to higher diversity of algae species, as predicted by the predation hypothesis. But the number of algae species decreased again when the snail population exceeded a limit, causing the extinction of the preferred food plants. The diversity of the prey, here the algae, reached a maximum under the influence of a medium population density of predators, here the snails. An interesting aspect of Lubchencos study is the extension of Paine’s predation hypothesis by including plants as prey species. In contrast to the study by Lubchenco (1978) Harper (1969) found that herbivores decrease plant diversity. This variable result may be due to the fact that herbivores can only increase the diversity of plants if they preferentially feed on the competitive dominant plant species, preventing them from displacing subdominant plant species. They decrease plant diversity if they prefer the subdominant plant species. In conclusion herbivores can potentially do both, increase or decrease plant diversity (Lubchenco 1978). This study focuses on the rich plant diversity in Namaqualand, part of the Succulent Karoo, a biodiversity hotspot. The Succulent Karoo is situated on the West Coast of South Africa and Namibia. Namaqualand is the part of the Succulent Karoo, which lies in South Africa. This area is a semi-desert to desert environment. The temperatures can reach over 40° in summer and can be below 0° in winter. During daytime the temperature fluctuations are also very high. The average annual rainfall is between 50 and 400 mm depending on the area (Cowling et al. 1999), with rain falling mainly in winter (June to August). Compared to other desert ecosystems Namaqualand has many unique biological features (Cowling et al. 1999). The rainfall is rare, but highly predictable (Desmet & Cowling 1999). Droughts are very rare and have a disastrous effect on the plants that are not adapted to it. There is nearly every year enough rain for plants to germinate, grow and to produce seeds that can establish successfully afterwards. Thus plants are not forced to invest in robustness or longevity, abilities Introduction 5 that are crucial in other deserts with droughts lasting for years. The high population turnover in plants in the Succulent Karoo, especially after droughts, is one reason for the high plant diversity here (Jürgens 1999). Usually, a semi-desert is not expected to be among the 25 most diverse places in the world. Connell (1964) suggested that diversity is associated with the stability within a system, because in a stable environment less energy is required for homeostasis. He pointed out that diversity increases if species have higher productivity due to more available energy. The Succulent Karoo however is not a very stable environment, at least not compared to equatorial rain forests. There are high temperature fluctuations during the day and rainfall is usually restricted to the winter months. Determining the reasons for the extraordinary high number of plant species in the Succulent Karoo can be of great importance for its conservation. If herbivore predators have a positive influence on plant diversity, it would be essential to include these animals in conservation programs for plants. The conservation of plants, and nature in general, is extremely important in this area because the plants are a tourist attraction and tourism is an important economic factor in Namaqualand. Furthermore, maintenance of plant biodiversity is also important for the local farmers, as livestock feeds on a large variety of plant species, mainly succulents and annuals. Grass is nearly absent in the Succulent Karoo, and a decrease of biodiversity (e.g. because of overgrazing) is characterized by an increase in abundance of unpalatable shrub species (i.e. Galenia africana, Todd & Hoffman 1999). Another reason why desert ecosystems are increasingly important for conservation is global warming. Because there is a strong likelihood of a rapid increase in temperature all over the world, the genetic stock held by desert ecosystems might be of enormous importance for mankind in the future (Cowling et al. 1999). The current rate of extinction is a loss we can ill afford. Additionally, the Succulent Karoo is an inadequately protected biome (Hilton-Taylor & Le Roux 1989). Botanically the Succulent Karoo is part of the Greater Cape Flora (Jürgens et al. 1991). The Succulent Karoo is home to about 1954 endemic plant species, making it the world’s richest succulent flora (Lombard 1999). Characterised by an open dwarf shrubland (Milton et al. 1997), the vegetation is dominated by leaf- Introduction 6 succulents and numerous species of highly abundant ephemeral geophytes flowering in spring (Cowling et al. 1999). Namaqualand harbours probably 10% of the succulent species in the world (Van Jaarsveld 1987). One of the most abundant families is the Mesembryanthemaceae (also called Aizoaceae), which are overwhelmingly concentrated in the Succulent Karoo (Hartmann 1991). In contrast to other winter rainfall-deserts, leaf succulents with a shallow rooting system dominate the vegetation. This rooting system makes the plants very vulnerable for droughts (Cowling et al. 1999). To grow in the cool wintertemperatures some plants have low temperature-optima for photosynthesis (Rossa & von Willert 1998). Namaqualand can be divided in smaller regions based on geology, topography, amount and season of rainfall. Goegap Nature Reserve, where this study was conducted, falls within the Hardeveld or Namaqualand Rocky Hills (Hilton-Taylor 1996) extending from Steinkopf in the north to Bitterfontein in the south. The vegetation cover here is relatively dense compared to other regions of Namaqualand. The landscape in the Hardeveld can be divided into hills (local name: koppies), plateaus and plains. Different soil features characterize each of these regions. On koppies there is usually shallow soil, as sand is continually removed by water. Wind and water deposit finer soil particles on plains and along drainage lines. The soil of plains is deeper and supports different plant communities. There are various species of small mammals occurring in Namaqualand. Some of them are endemic. In fact 85% of South Africa’s endemic mammal species are small (Gelderblom 1995). Especially in the Hardeveld, the region of Namaqualand where this study was conducted, the population densities of small mammals can be extraordinary high (Schradin & Pillay 2005; Jackson 1999), while large mammals are relatively rare in species and numbers. Most of the small mammals are muroid rodents, like the striped mouse (Rhabdomys pumilio), the bush-Karoo-rat (Otomys unisulcatus) and several species of gerbils. But elephant shrews and mole rats are also represented with some species. There are no obvious reasons for the extraordinary number of plant species in the Succulent Karoo, but several hypotheses might apply. The plants in this area are food for many herbivores. Thus, the plants can be regarded as prey and the herbivores as their predators or consumers. The present study investigates if Introduction 7 the predation-hypothesis can be applied to the Succulent Karoo, and if the presence of small mammals as the dominant plant predators can at least partly explain the high number of plant species. It is suggested that small mammals of the Succulent Karoo have an influence on the plant diversity in their surroundings. According to Andrews and O`Brien (2000) small mammal distribution is representative for the distribution of mammals in general. Thus, bigger herbivores such as zebras and antelopes were not included in this study. Furthermore, small mammals have the advantage of a small range of action in comparison to larger mammals, like ungulates. This is highly useful for a study like this where small-scale differences in species assemblage and population densities were investigated. In detail, I tested the following hypotheses: 1. The presence of small mammals as plant predators correlates with plant diversity. 2. Small mammals as plant predators affect competition between plant species by preferably feeding on dominant or subdominant plant species. According to Lubchenco (1978) herbivores increase plant diversity if they prefer dominant plant species. In this case they provide space for subdominant species that would otherwise be outcompeted. 3. The effect of small mammals on plant diversity is supposed to be greatest in the direct surrounding of their nests, especially in case of central place foragers like O. unisulcatus (bush-Karoo rats). This would predict differential diversity around occupied nests of bush-Karoo rats compared to abandoned areas. While small mammals might have an influence on plant diversity, the evolution and maintenance of plant biodiversity is very likely also dependent on many other factors. While the focus of this study was on the role of small mammals in this process, data regarding several other ecological factors were collected, as well. In the first place some edaphic (soil concerning) parameters were investigated that are hypothesised to have a great influence on species diversity. The focus was on soil nutrients. The concentration of the ions from sodium, potassium, calcium, manganese, magnesium and iron were measured. In Introduction 8 addition the pH-value in the soil solution was measured. The nutrient status and pH are important edaphic factors for characterisation of Succulent Karoo soil (Lechemere-Oertel & Cowling 2001). Curiously, Succulent Karoo seedlings accumulate more biomass if they grow in nutrient-poor soil in comparison to nutrient-rich soil (Lechemere-Oertel & Cowling, 2001). Another recorded factor was altitude. In the Andean forests, for example, there is a general tendency for plant diversity to decrease with increasing altitude (Gentry 1988). These data on edaphic parameters were used for descriptive statistical analyses (Cluster analysis) to create hypotheses and predictions for future research into the understanding of plant biodiversity in the Succulent Karoo. In addition a possible impact of large ungulate herbivores on plant- and small mammal diversity was investigated. The history of grazing by domestic animals in Namaqualand dated back more than 2000 years (Smith 1999). The keeping of livestock especially goats and sheep is common in the Succulent Karoo, which might have a great influence on this biome. According to the predation-hypothesis it can be expected that, like small mammals, livestock might have an influence on plant diversity. Maybe very high stocking rates have a negative effect on small mammal diversity because overgrazed rangelands show a lack of cover for predator avoidance (Joubert & Ryan 1999). In this context Milton et al. (1994) found that there is a progressive degradation of rangelands resulting in irreversible changes in diversity and abundance of Karoo vegetation. Although several studies found no differences in plant diversity between a reserve and a lightly grazed, neighbouring farm (Todd & Hoffman 1999 inter alia), I investigated this topic again by comparing plant biodiversity between a farm, which had a history of overgrazing by livestock, and a nature reserve. I tested the following prediction: 4. There is an effect of grazing on the number of plant/small mammal species. Subjects, materials and methods 9 3. Subjects, Materials and Methods 3.1. Study area The study was conducted in June-December of 2004 in Goegap Nature Reserve. This reserve is near the town Springbok in the northwest of South Africa (Northern Cape). Goegap Nature Reserve is situated in the middle of Namaqualand, which is part of the Succulent Karoo. It is situated in the Namaqualand Rocky Broken Veld (Acocks 1988) also called Hardeveld (Hilton-Taylor 1996). The area is semiarid. Rain falls mainly in winter and the annual average is 160mm (Rösch 2001). The vegetation type is Succulent Karoo (Milton et al. 1997), dominated by leaf succulent shrubs and many ephemeral species, mainly flowering in spring. This biome is a bioregion of exceptional succulent plant diversity and endemism (Hilton-Taylor 1996). Within Goegap Nature Reserve ten areas differing with regards to structural and floristic features were chosen for the main project (s. 3.3.). To avoid a bias in the dataset it was important to choose more or less homogenous places, which were ecologically different from each other. The areas were designed to be representative for the plant assemblage of five different management units that were identified in Goegap Nature Reserve by Rösch (2001). The distribution of the ten areas is showed on the map (Fig. 1) while their characteristics are listed in Table 1. Subjects, materials and methods 10 Figure 1: Goegap Nature Reserve with the ten investigated areas, the neighbouring farm and two public roads. The main road of the reserve is also shown. The map shows the distribution of the ten areas in the reserve. The two transects where the Fence line (s. 3.6.) was conducted are marked with an F. Reference: Software MapSource, South Africa Table 1: Overview about the ten investigated areas; Rainfall: 0-50mm/year = sparse, 100150mm/year = moderate, >150mm/year = plenty; all edaphic factors, altitude, soil texture and plant cover: results from this study (s. 4.1.3.) organic soil components in % referring the dry weight; soil: + = yes, - = no soil altitude Areas (m o. NN) rocky 1 954.2 + 2 935.96 + 3 934.9 + 4 895.35 + 5 855.3 + 6 873.63 + 7 901.85 + 8 977.3 + 9 1062 + 10 1109.85 - sandy + + + plant cover (%) Edaphic factors organic soil c (Na) c (K) c (Mg) c (Ca) mg/l mg/l mg/l rainfall Winter Summer components (%) mg/l moderate 10 10 2.20 5.15 13.01 5.89 48.14 moderate 1 5 0.93 1.16 5.78 4.70 13.72 sparse 0 1 0.97 2.68 10.81 3.10 11.24 sparse 40 25 0.44 1.03 1.89 1.94 7.23 sparse 40 45 2.27 65.68 23.40 23.85 95.51 sparse 0 1 0.98 10.78 10.26 2.87 8.95 moderate 35 40 1.47 7.54 4.53 5.50 86.12 moderate 50 60 1.69 4.10 6.09 2.37 14.54 plenty 40 50 2.28 1.94 2.15 3.48 17.54 plenty 80 50 2.95 3.70 1.26 6.79 33.67 Subjects, materials and methods 11 3.2. Animals Ten different small mammal species were trapped during the study. The only exclusively diurnal species was the striped mouse (Rhabdomys pumilio). The striped mouse is a muroid rodent (Brooks 1982; Dewsbury & Dawson 1979) with an adult mass range of 40-85g (Schradin 2003) in both sexes. It occurs in many parts of southern Africa, including grasslands, marsh, forest, desert and the Succulent Karoo (Kingdon 1974). In the Succulent Karoo R. pumilio lives in social groups of one breeding male, 1-4 breeding females and their nonreproductive, adult offspring (Schradin & Pillay 2003). Group-members sleep together in one nest (Schradin & Pillay 2004), built in bushes. Sometimes they occupy old nests of bush Karoo rats (Schradin in press). In spite of their group-living organisation striped mice forage alone during the day (Schradin in press). Their diet is omnivorous and contains herbage, fruits, insects and seeds (Curtis & Perrin 1979; Kerley 1992). The bush-Karoo rat (Otomys unisulcatus) is a rodent endemic to the subarid regions of southern Africa (Skinner & Smithers 1990). It is confined to the Karoo region of the South West Arid Zone (Davis 1974; Skinner & Smithers 1990) and is often associated with the courses of ephemeral streams and rivers (Shortridge 1934; Diekmann 1979). O. unisulcatus is a medium sized rodent with an adult mass range of 70-135g (Pillay 2001). It has a shaggy pelage, which is ash grey dorsally and buff white ventrally. (Pillay 2001) Its nest is a large stick lodge up to 1.0m in diameter and up to 1.5m high (Schradin in press). It is usually situated at the base of shrubs such as Zygophyllum retrofractum or under large rocks (Dieckmann 1979, pers. observ.). They are constructed of sticks, twigs and flowering ephemerals, sometimes enclosing the shrub completely (Dieckmann 1979; Plessis & Curley 1991). Passages are made within the dense shrub. A small nest of finer material is constructed at the centre of the large structure in a shallow burrow or depression. They always contain some green material if the nest is occupied (Dieckmann 1979, pers. observ.). The literature disagrees concerning the activity period of the bush Karoo rat. It is described as diurnal (Plessis & Kerley 1991, Plessis et al. 1990) or as crepuscular (inter alia Skinner & Smithers 1990). O. unisulcatus is exclusively herbivorous (Kerley 1992; (Brown & Willan 1991). Shrubs dominate their diet (Plessis et al. 1990). The feeding on highly hydrated plant material is critical to the rat’s survival (Brown & Willan 1991). Mostly the Subjects, materials and methods 12 litters consist of 2 semi-precocial young that are weaned as early as eight days of age (Pillay 2001). Interestingly the bush Karoo rat is not physiologically well adapted to the arid environment it lives in (Du Plessis et al. 1989; Pillay et al. 1994). O. unisulcatus uses behavioural adaptations to cope with xeric conditions such as building stick lodges and feeding on succulent plants to get access to water. One of the numerous nocturnal species was the Namaqua-Rock mouse (Aethomys namaquensis). A. namaquensis is restricted to the central Karoo in the Northern Cape Province. This rat like rodent has an average body mass of 50g. Its fur is reddish brown to yellowish light brown with a white underpart. The tail is longer than the body and happens to have dandruffs. A. namaquensis lives in small colonies in rocky areas, where they hide in nests under rock crevices or in burrows under shrubs. Their diet consists of seeds from grass and other plants. A. namaquensis gives birth to 3-5 pups in summer month (Stuart &Stuart 2001). The smallest of the trapped species was the pygmy mouse (Mus minutoides). This mouse is widely spread over different habitats in southern Africa and weighs just about 6g. Its fur is grey to reddish brown with a white underpart. Not much is known about the social behaviour of this species. In this study more than one animal was trapped in tha same place mostly. more than one animal in one place. The birth weight of the up to seven pups is under 1g. They are often born in self-dug burrows, but deserted burrows from other species or other hideouts are also used (Stuart &Stuart 2001). Three species of Gerbils were trapped, two pygmy gerbils (Gerbillurus vallinus, Gerbillurus paba) and the short tailed-gerbil (Desmodillus auricularis). The last is much heavier than the pygmy gerbils weighing about 50g compared to 25-35g. D. auricularis also has got a tail which is shorter than the body, which is most uncommon for gerbils. The fur colour of the three species is nearly equal varying from reddish brown to grey. All species have a white belly. In contrast to the other species D. auricularis has got white patches at the base of its ears. The diet of gerbils consists of seeds and sometimes insects. All these gerbils occur in dry, sandy areas with G. paeba being most common. These nocturnal animals dig their own burrows. G. vallinus lives in colonies whereas G. paeba build small groups and D. auricularis is solitary or monogamous (Stuart &Stuart 2001). Subjects, materials and methods 13 In addition to all these rodents three elephant shrew species were trapped (order Macroscelidae). Macroscelidae belong to the clade Afrotheria, which also includes aardvarks, elephants, hyraxes, golden moles, tencres and serenians (Murphy et al. 2001). The round-eared elephant shrew (Macroscelides proboscidreus) has a long pelage that is light grey-brown on the dorsum, yellowbrown on the flanks and white on the ventrum (Corbet & Hanks, 1968). M. proboscideus has long slender legs and a long, narrow, semi-flexible snout (Nowak 1991). The average adult body mass is 45g. M. proboscideus is endemic to Africa and native to Namibia, southern Botswana and the Cape Province of South Africa (Corbet & Hanks 1968; Nowak 1991). According to Nowak (1991) the round-eared elephant shrew is mainly diurnal and just sometimes crepuscular or nocturnal. In this study no elephant shrew was ever trapped during the day. So it is likely, that the animals switched their schedule to avoid the threat of diurnal predators like birds of prey (i.e. jackal buzzards, Buteo rufofuscus), which are common in the study area. Their diet is dominated by insects along with roots, berries (Nowak 1991), herbage and seeds (Kerley 1992). It lives on sandy and thornbrush plains and seeks shelter in burrows under bushes (Nowak 1991) or rock crevices (Dieckmann 1979). The animals breed in August and September. M. proboscideus is mainly solitary according to Nowak (1991), but it is also suggested to be monogamous (Rathbun pers. commun.) Once a year 1-2 precocial young are born, that are nearly immediately able to move. They are weaned at 16-25 days and reach sexual maturity after about 43 days (Rathbun & Fons). The two Rock-elephant shrew species, the Smith-Rock-Elephant shrew (Elephantulus rupestris) and the Cape-Rock-Elephant-shrew (Elephantulus edwardii) are more common in rocky areas as the name implies. There are heavier than M. proboscideus, weighing 65g and 50g, respectively. These two species are difficult to distinguish from each other in the field. In contrast to E. edwardii, E. rupestris has a clear white eye ring, a larger brown pelage at the neck, and a very hairy tail. They have various fur colours (Stuart &Stuart 2001). In other aspects they are similar to M. proboscideus. Subjects, materials and methods 14 3.3. Correlation between small mammals and plants 3.3.1. Trapping To determine the diversity of small mammals, trapping was performed at the ten different sites. Thirty locally hand-made Shearman-traps (26 x 9 x 9cm) were placed 10m apart, in a line. The trapping transects were 290m long. Each Trap contained bait and a piece of cotton wool, to avoid trap-death caused by frost. The bait was a mixture of bran flakes, currants, sea salt, salad oil and peanutbutter. Prebaiting was done for two days in the afternoon before trapping. One day before trapping, no prebaiting was performed. Small mammals were trapped in each area for four days. On the first two trapping days trapping occurred in the afternoon, three hours before and three hours after sunset followed by a day without trapping. On the last two trapping days trapping was performed in the morning three hours before until three hours after sunrise. This time schedule was chosen in order to trap the diurnal as well as the nocturnal animals. Traps were checked every 90min to avoid the occurrence of trap deaths and to open traps for other small mammals present at the transects. Trapped animals were marked individually with hair dye (method from Weiß et al. 1996), weighed and sexed. Trapping was done twice, July/August (winter) and the second one during October/November (summer). No trapping was performed in the rain or after a temperature drop of more than 5°C from one night to the other. 3.3.2. Vegetation survey Vegetation analyses were performed after first trapping in August and before second trapping in October. The surveys were distributed evenly along the trapping-transect. At each transect, plant species in and around five squares (each 2x2m) were determined. All plant species present, the number of individuals per species and the ground cover was noted. The mean ground cover in five vegetation surveys was used for statistics (s. 3.2.7.)The seedlings found in spring were not classified, as they were not present during the preceding trapping session. Plants were divided in annual and perennials species. The number of individuals was categorized as follows (after the Braun-Blanquet Method as described Wilmanns 1998). Subjects, materials and methods r= 1 - 2 Individuals += 3 - 10 Individuals 1= 11 - 100 Individuals 15 2m = > 100 Individuals If one species covered more than 5% of the ground the following classification was used. 2a = 5 - 12,5 % 2b = 12,5 - 25 % 3= 25 - 50 % 4= 50 - 75 % 5= > 75 % Figure 2: Field assistant performing a vegetation analysis in a 2x2m square in area 8 Subjects, materials and methods 16 3.3.3. Soil samples Three samples of soil (100g) were collected in each area from a depth of 3cm. Samples were taken on open positions, not under shrubs or rocks. Collection took place after a period without rainfall in the beginning of November. The parameters pH-value and elemental composition (Na +, K +, Mg2+, Fe+, Mn2+) were measured. The soil was air dried and sifted through a mesh with a mesh-size of 2mm. To measure pH-value 10g of soil from each area was mixed with 25 ml dH2O and measured with a pH-Meter (pH 91, WTW). For the determination of the elemental composition with an AAspectrometer (Unicom 939), an extract from 2.5g soil was produced. Two extracts and three blind tests were analysed for each area. The soil was mixed with 50ml NH4Cl (1mol/l) and left untouched for 4h. Afterwards the extracts were shaked for 2h on an automatic shaker (Schüttelmaschine LS20, Gerhardt) and left untouched over night. The extract was filtered. To measure Ca and Mg 10ml of extract was mixed with 1ml buffer (7.6g KCl + 2.5ml 37%HCl in 200ml dH2O.). For the measurement of Fe and Mn, three standards were prepared with 1ppm, 2ppm and 5ppm of Fe and Mn. For the measurement of the other elements, four standards were prepared with the following composition: 1. 1ppm Na, 2ppm K, 2ppm Ca, 0.1ppm Mg 2. 2ppm Ca, 5ppmCa, 5ppm K, 0.2ppm 3. 5ppm Na. 10ppm K, 10ppm Ca, 0.5ppm Mg 4. 10ppm Na, 20ppm K, 20ppm Ca, 1ppm Mg Standards were used to calibrate the AAspectrometer. Samples were diluted if necessary to fit in the range of the standards. Extracts were measured with a Unicom 939 AAspectrometer. To measure the percentage of organic components in the soil, two samples (≈ 4g) were dried in a cabinet desiccator (Heraeus thermicon P) over night, weighed (Mettler Type AE 163, accuracy of 0,001g ), cooled down to 20° in an Exicator and stored in a muffle furnace (Heraeus Function line Typ 12) for 15h. In the first 2h the oven heated the samples up to 400° temperature remained here for 3h. Than the oven heated the samples up to 600° in another 2h and kept them on this temperature for 6h. Afterwards the samples needed 2h to cool down to 20° Subjects, materials and methods 17 and were weighed again. The weightloss in reference to the weight after drying was calculated. 3.3.4. Altitude The altitude of each transect in the ten areas was measured with a GPS (eTrex Venture, GARMIN International, USA) at the start- and end-point. The mean value was used for statistics (s. 3.3.7.). 3.3.5. Rainfall The mean value of rainfall from the years 1998 to 2002 was taken (information provided by Goegap Nature Reserve) with nine gauging stations distributed in the whole Nature Reserve. The rainfall in the trapping areas was assumed according to the nearest gauging station. No measurements had been taken in the years 2003 and 2004. 3.3.6. Statistics Correlations between the number of plant species and the number of small mammal species and total number of small mammals were calculated as the Spearman-Rank-correlation coefficient (rs). Results were corrected with Bonferroni. The influence of the number of small mammal species, the total number of trapped animals, the soil characteristics, and the altitude on plant diversity were calculated with a general linear model with the program Statistika. First all data were tested for their normal distribution with a KS normality test. The data did not differ significantly from the normal distribution. Plant diversity was taken as dependent factor and all other factors were considered independent. No interactions between the factors were taken into account. In a step-wise procedure the independent factors with the highest p-value were left out one by one until all remaining factors were significant. To compare the different areas a Cluster-analysis was calculated. The following factors were included in the Cluster-analysis for winter and summer: small mammal number, small mammal species number, ground cover and number of plant species. In addition the altitude and some soil characteristics were included, as there were, the percentage of organic components and the Subjects, materials and methods 18 concentration of the ions Na+, K+, Mg2+, Ca2+. The statistic program SPSS was used. Differences were considered significant if their probability of occurring by chance was less than 0.05 (two tailed). 3.4. Food-preference tests This experiment was performed with two species, bush-Karoo-rats (O. unisulcatus) and striped mice (R. pumilio). Ten rats and eleven mice were tested. Striped mice were chosen as a representative, medium sized, diurnal species (Schradin, Pillay 2004) and bush-Karoo-rats were chosen as a representative, larger, crepuscular species (Brown & Willan 1991). Animals were trapped for food-preference-tests as described above. Trapping was performed in Goegap Nature Reserve and, in case of bush-Karoorats, on the neighbouring farm. Trapped animals were sexed, weighed and marked with hair dye and/or eartags to identify them in case of recapture. Each animal was used only once. The animals were put into a cage with sand as substrate and pieces of six plant species. The plant pieces were all 3cm long but of different weight, because equating weight would have led to immense differences in surface and length (some plant species were succulent). It was assumed that similar length ensures similar conditions for each plant species better than similar weight. An equal number of dominant and subdominant plant species were provided. The determination of dominance of plant species in areas were the species lived was based on the previous studies (s. 3.3.). Plants were freshly collected and stored in a refrigerator in airtight plastic bags containing wet tissue paper. Plant species were arranged in a row on the short side of the cage. Dominant species altered with subdominant species. Order was changed systematically every experiment. The animals were left alone in a quiet room. Beginning at 1h, the cage was checked every 30min. The experiment was terminated when visible damage was done to one or more plant pieces, or a maximum of 4h had elapsed. Tissue paper was positioned on top of the cage to provide cover. Immediately after the experiment animals were released at the place of capture. Plant species were weighed (Mettler Type AE 163, accuracy of 0,001g) before and after testing to determine the weight loss. To consider the weight loss due to evaporation eight controls experiments were conducted. Here the same procedure was used, but without an animal in the cage. The median of Subjects, materials and methods 19 the eight control experiments was subtracted from the measured weight loss in all experiments. In the event of negative results due to this subtraction the weight loss was assumed to be 0. Tests were performed during the active period of the species. In the first set of tests Euphorbie spec., Mesembryanthemum guerichianum and Leipoldtia pauciflora were used as dominant plant species for O. unisulcatus, Osteospermum sinuatum, Cheiridopsis denticulata and Aptosimum spinescens were used as subdominant plant species. For R. pumilio Zygophyllum retrofractum, Lycium cinereum and Leipoldtia pauciflora were used as dominant plant species and Galenia africana, Lessertia dfiffusa and Rhus spec. as subdominant species. Because the first set of tests (pilot study) revealed that a mixture of perennial and annual plant species can cause a bias in the dataset, a second set of tests was performed, including only perennial plant species. In the second set of tests two plant species were left out for each small mammal species. O. unisulcatus was tested with Euphorbie spec, and Leipoldtia pauciflora as dominant plant species and Osteospermum sinuatum and Aptosimum spinescens as subdominant plant species. R. pumilio was tested with Zygophyllum retrofractum and Leipoldtia pauciflora as dominant plant species and Galenia africana and Rhus spec. as subdominant species. The weight loss of the dominant/subdominant plant species in percentage referred to the weight at the beginning of the experiment was added. Dominant species were compared to subdominant species with the Wilcoxon matched pairs rank sign test. For in depth analyses, the Friedman test for multiple paired comparisons was used, followed by a Wilcoxon-Wilcox test as post test. 3.5. Plant biodiversity around occupied and unoccupied bush Karoo rat nests The vegetation around occupied nests of bush-Karoo-rats (Otomys unisulcatus) was compared to that around unoccupied nests. Always one occupied and one unoccupied nest in areas as similar to each other as possible were investigated. The two nests of each pair were situated as close to each other as it was possible to ensure that the inhabitants of the occupied nest did not influence the area around the unoccupied one. Changes in the landscapes (i.e. riverbeds, rocks) were taken into account. One pair was situated on an adjoining farm (cf. 3.6.); all Subjects, materials and methods 20 other pairs (11) were in Goegap Nature Reserve. The diversity of plant species around the nests was compared with the Wilcoxon matched pairs rank sign test. The sample size was twelve pairs. Occupied nests were identified by fresh droppings and fresh plant material, as indicators. Vegetation survey was performed in a 10m radius around the nests. The same methods for data recording were used that were described above (cf. 3.3.3.). 3.6. Fence line The number of small mammal species on both sides of a fence between a farm and the Goegap Nature Reserve was determined (Fig. 1). The farm has not been used for stocking for two years, but great differences relative to the nature reserve were still obvious in the assemblage of plant species. Trapping was performed in a 290m transects as described above. The two trapping transects were situated parallel to the fence. To exclude an influence of the fence itself and to avoid trapping small mammals from the nature reserve on the farm or vice versa, that were attracted by the traps themselves, traps were placed 20m away from the fence. Figure 3: Fence between Goegap Nature Reserve (left side) and a neighbouring farm (right side) Subjects, materials and methods 21 Eleven vegetation analyses on each side were performed to compare the plant diversity. The vegetation analysis was done as described above. Squares were distributed almost evenly along the transects. The numbers of plant species in the eleven pairs of squares were compared with the Wilcoxon matched-pairs signed-rank test. The number of small mammal species and the number of trapped small mammal individuals were compared descriptively as sample sizes were too small for statistics. Results 22 4. Results 4.1. Correlation between small mammals and plants 4.1.1. Comparison between winter and summer Significantly more plant species were found in summer (p=0.007; T=1). The comparison between the plant cover of summer and winter revealed no significant difference (p=0.514; T=17). 70 small mammal individuals were trapped in winter compared to 119 animals in summer (p=0.007; T=0). In the first trapping season (August/November) ten species of small mammals were trapped. In the second trapping season (October/November) nine species of small mammals were trapped. The species were the same as in winter without the brush-tailed Hairy-footed Gerbil (G. valinus). There was no significant difference concerning the number of species (p=0.334; T=4). The detailed trapping data from winter and summer can be found in Tables 4 and 5 (Appendix). Results 23 Table 2: Summary about the trapped small mammal species in the ten investigated area, their number (sM-species) and the number of trapped individuals of all species combined (sMnumber). First value stands for winter, second for summer. Area sM-species sM-number G. peaba 1 2 3 2 2 2 5 6 8 12 4 10 3 4 3 2 2 4 2 7 2 1 5 1 5 6 7 8 9 1 1 4 4 1 2 4 6 2 9 17 12 8 12 28 26 2 9 11 7 4 8 10 10 4 4 15 16 G. valinus 1 1 D. auricularis 2 12 2 R.pumilio 1 6 2 1 M. minutoides A. namaquensis 1 6 2 O. unisulcatus 2 3 7 8 2 8 M. proboscideus E. edwardii E. rupestris 1 5 3 4 3 2 2 4 2 1 4 2 2 3 2 Results 24 4.1.2. Winter trapping season The number of plant species in the ten investigated areas showed a significant positive correlation with the total number of trapped animals (p=0.032; rs= 0.732; Fig. 4) and with the number of small mammal species occurring there (p=0.004; rs=0.895; Fig.5). Winter 25 plant species total 20 15 10 5 0 0 2 4 6 8 10 12 14 16 18 sM-number Figure 4: Correlation between the number of plant species and the total number auf trapped small mammals (sM-number) in ten different areas. Spearman-Rank-Correlation-test: p=0.032; rs= 0.732 Winter 25 plant species total 20 15 10 5 0 0 1 2 3 4 5 sM-species Figure 5: Correlation between the number of plant species and the number auf occurring small mammal species (sM-species) in ten different areas. Spearman-Rank-Correlation-test: p=0.004; rs=0.895 Results 25 93.1% of the plant species in winter were perennial. The number of perennial plant species showed a nearly significant correlation with the total number of small mammals (p=0.056; rs=0.713; Fig. 6) and a significant correlation with the number of small mammal species (p=0.002; rs=0.882; Fig. 7). A correlation between small mammals and annual plant species was not calculated, because they were nearly absent in winter. In half of the areas there were no annuals at all and in the other areas there were only one or two species. Winter 25 perennial plant species 20 15 10 5 0 0 2 4 6 8 10 12 14 16 18 sM-number Figure 6: Correlation between the number of perennial plant species and the total number of small mammals (sM-number) in ten different areas. Spearman-Rank-Correlation-test: p=0.056; rs=0.713 Results 26 Winter 25 perennial plant species 20 15 10 5 0 0 1 2 3 4 5 sM-species Figure 7: Correlation between the number of perennial plant species and the number of occurring small mammal species (sM-species) in ten different areas. Spearman-RankCorrelation-test: p=0.002; rs=0.882 The red point represents two areas with the same values. 4.1.3. Summer trapping season The total number of plant species did neither correlate with the number of small mammal species (p=0.12; rs=0.559; Fig. 8) nor with the total number of trapped small mammals (p=0.598; rs=0.334; Fig 9). Summer 40 plant species total 35 30 25 20 15 10 5 0 5 10 15 20 25 30 sM-number Figure 8: Correlation between the number of plant species and the total number of trapped small mammals (sM-number) in ten different areas. Spearman-Rank-Correlation-test: p=0.12; rs=0.559 Results 27 Summer 40 plant species total 35 30 25 20 15 10 5 0 1 2 3 4 5 6 7 sM-species Figure 9: Correlation between the number of plant species and the number of occurring small mammal species (sM-species) in ten different areas. Spearman-Rank-Correlation-test: p=0.598; rs=0.334 The red point represents two areas with the same values. There was no significant correlation between perennial plant diversity and the total number of trapped small mammals (p=0,12; rs =0,612; Fig. 10). However the number of perennial plants species and the number of small mammal species showed a significant positive correlation (p=0.03; rs=0.763; Fig. 11). Compared to the winter there were considerable more annual species in this season (Wilcoxon matched pairs rank sign test; p=0,005 t=0). There was no significant correlation between annual plant species and small mammals, neither with the number of small mammal species (p=0.859; rs= 0.032) nor with the total number of small mammals (p=0.815; rs= -0.110). Results 28 Summer 25 perennial plant species 20 15 10 5 0 0 5 10 15 20 25 30 sM-number Figure 10: Correlation between the number of perennial plant species and the number of trapped small mammal (sM-number) in ten different areas. Spearman-Rank-Correlation-test: p=0.12; rs=0,612 Summer 25 perennial plant species 20 15 10 5 0 0 1 2 3 4 5 6 7 sM-species Figure 11: Correlation between the number of perennial plant species and the number auf occurring small mammal species (sM-species) in ten different areas. Spearman-RankCorrelation-test: p=0.03; rs=0.763 Results 29 4.1.4. Correlation between plant cover and small mammals The Spearman-Rank-Correlation-test revealed that plant cover correlated with the number of trapped small mammals in the ten areas, as well as with the number of trapped small mammal species. The correlations were significant for winter (plant cover–sM-number: p=0.014, rs=0.741; plant cover–sM-species: p= 0.004, rs=0.811) and summer (plant cover–sM-number: p=0.002, rs=0.844; plant cover – sMspecies: p= 0.038, rs=0.660). 4.1.5. Soil survey Data of the soil survey are included into the cluster-analyses (4.1.6) and here described descriptively. The pH-values of the ten areas had a range from 5.7 to 8.5 (Fig. 12). The lowest pH-values were found in the highest areas (9, 10). Soil survey 10 8 pH 6 4 2 0 0 1 2 3 4 5 6 7 8 9 10 11 Areas Figure 12: pH-value of the ten areas, measured with dH2O as solvent. Results 30 The percentage of organic component in the soil samples had a range from 0.92 to 2.97 % (Fig. 13). Soil survey 3,5 Organic components in % 3,0 2,5 2,0 1,5 1,0 0,5 0,0 0 1 2 3 4 5 6 7 8 9 10 11 Areas Figure 13: Organic components of soil samples from the ten areas in % from the oven-dry mass. The median from 2 samples is given. Results 31 The concentration of Fe2+ was too low to be measured. Mn2+ concentrations were also very low and had a range from 0.076 mg/l to 0.382 mg/l. The concentrations of the other ions are given in Figure 14. Soil survey Concentration of Ions in mg/l 100 Natrium Calcium Magnesium Kalium 80 60 40 20 0 0 1 2 3 4 5 6 7 8 9 10 11 Areas Figure 14: Concentration of the ions from Na, Ca, Mg, K in the soil of the ten areas. Mean value of two soil solutions is given. Results 32 4.1.6. Cluster-analysis The following factors were included in the Cluster-analysis for winter and summer: small mammal number, small mammal species number, ground cover and number of plant species. In addition the altitude and some soil characteristics were included, as there were, the percentage of organic components and the concentration of the ions Na+, K+, Mg2+, Ca2+. Three Clusters were identified with a hierarchic Cluster-analysis. Areas 8, 9 and 10 belonged to the first cluster, area 5 stood alone in the second one and areas 1, 2, 3, 4, 6 and 7 were in the third cluster. The areas in the first cluster were characterised by high values concerning small mammals and plants, whereas the second cluster (area 5) had very high concentrations of soil nutrients. The areas in the third clusters had moderate values in most of the factors (Tab. 3). 4.1.7. General linear model The general linear model for winter revealed a significant result (p<0.05, F=5.87). Floral diversity was explained by the number of small mammals (p=0.0006, F=29.86) and by the concentration of Manganese in the soil (p=0.0034, F=16.94). The model for summer was also significant (p<0.0001, F=48.27), and the only remaining significant factor explaining plant diversity was the number of small mammals species (p=0.000008, F=81.31). Results Cluster - analysis Table 3: Mean value and standard deviation of all factors included in the hierarchic cluster-analyses. Cluster 1 includes the areas 8, 9 and 10. Cluster 2 includes area 5 alone and Cluster 3 consists of the areas 1, 2, 3, 4, 6 and 7. Winter number of 2 plant number of sM- sM- plant Altitude (m) organic c (Na) c (K) c (Mg) c (Ca) particles (%) mg/l mg/l mg/l mg/l n=3 Mean 18.33 14.67 4.00 56.67 25.33 23.33 4.67 53.33 1049.72 2.31 3.25 3,17 4.21 21.91 SD 4.51 2.52 0 20.82 5.77 6.43 1.15 5.77 67.12 0.63 1,15 2.57 2.30 10.29 n=1 Mean 9.00 2.00 1.00 40.00 11.00 8.00 1.00 45.00 855.30 2.27 65,68 23.40 23.85 95.51 . . . . . . . . . . . . n=6 Mean 8.67 4.00 1.50 14.33 21.00 6.83 1.67 13.58 915.98 1,16 4,72 7.71 4.00 29.23 SD 5.54 3.52 1.38 18.40 10.32 5.00 0.82 15.77 30.45 0.60 3,88 4.29 1.59 31.79 SD 3 sM- Edaphic factors plant species number species cover (%) plant species number species cover (%) Cluster 1 sM- Summer . . 33 Results 34 4.2. Food-Preference-tests 4.2.1. Pilot study 4.2.1.1. Striped mouse (R. pumilio) The Friedman-test revealed an overall significant difference in how much was eaten from the four different plant species (p=0.0001). The following WilcoxonWilcox-test showed that Lycium cinereum (dominant) and Lessertia diffusa (subdominant) are different from Leipoldtia pauciflora (dominant) and Rhus spec. (subdominant) concerning the consume by R. pumilio. The other plant species were not different from each other. 4.2.1.2. Bush-Karoo rat (O.unisulcatus) The Friedman-test revealed an overall significant difference in how much was eaten from the four different plant species (p=0.004). However the following Wilcoxon-Wilcox-test showed no differences between certain plant species. Figure 15: bush-Karoo rat (Otomys unisulcatus) with ear tag in a cage in which the foodpreference test were performed Results 35 4.2.2. Second set of tests 4.2.2.1. Striped mouse (R. pumilio) R. pumilio showed a significant food preference for the dominant plant species Z. retrofractum and L. pauciflora in comparison to subdominant plant species G. africana and Rhus spec. (data for dominant species and subdominant species combined; Wilcoxon matched pairs rank sign test; T=6; p = 0.007). The median percentage of eaten dominant plants was 18.10 and 11.04 for subdominant plants, respectively (Fig. 16). Food-preference-test R. pumilio 50 eaten plant pieces in % 40 30 20 10 0 dominant plants subdominant plants Figure 16: Comparison of the quantity R. pumilio ate from two dominant /subdominant plant species provided in a food-preference-test. Data were corrected for evaporation by control experiments. t=6; p = 0.007 The Friedman-test revealed an overall significant difference in how much was eaten from the four different plant species. The following Wilcoxon-Wilcox-test showed that Z. retrofractum is different from all other plant species concerning the consume by R. pumilio. The dominant shrub Z. retrofractum was the preferred food-plant. The other plant species were not different from each other. Results 36 4.2.2.2. Bush Karoo rat (O. unisulcatus) O. unisulcatus showed a significant food preference. In contrast to the striped mice they preferred the subdominant plants Osteospermum sinuatum and Aptosium spinescens to the dominant plant species L. pauciflora and Euphorbia spec. (data for dominant species and subdominant species combined; Wilcoxon matched pairs rank sign test; T=1; p = 0.002). The median percentage of eaten subdominant plants was 95.33 and 3.41 for dominant plants (Fig. 17). Food-preference-tests O.unisulcatus 140 eaten plant pieces in % 120 100 80 60 40 20 0 dominant plants subdominant plants Figure 17: Comparison of the quantity of dominant and subdominant plants eaten by O. unisulcatus provided in a choice test. Data were corrected for evaporation by control experiments. T=1; p = 0.002 The Friedman-test revealed an overall significant difference in how much was eaten from the four different plant species. The following Wilcoxon-Wilcox-test showed that O. sinuatum is different from all other plant species concerning the consume by O. unisulcatus. The other plant species were not different from each other. O. sinuatum was the preferred food-plant. Results 37 4.3. Plant biodiversity around bush Karoo rat nests The effect of bush-Karoo rats on their direct environment was investigated by counting the plant species in a circuit of 10m around occupied and unoccupied bush-Karoo rat nests. The comparison between the surrounding of occupied and unoccupied bush-Karoo-rat nests (Fig. 18) showed a significant difference concerning the number of plant species found in a circuit of 10m. Significantly more plant species were found around occupied nests (Wilcoxon matched pairs rank sign test; T=16.5; p = 0.05). . BKR-nests 30 number of plant species 25 20 15 10 5 0 unoccupied occupied Figure 18: Comparison between the number of plant species in a circuit of 10m around occupied and unoccupied nests of O. unisulcatus. Median and original data are given. A line connects the data of one pair of nests in the same area. T=16.5; p = 0.05 Results 38 4.4. Fence line A significant difference concerning the plant diversity in Goegap Nature Reserve and the farm was found (Wilcoxon matched pairs rank sign test T=2.5; p = 0.01; Fig. 19). In nine of eleven pairs there were more plant species on the farm. Median number of plant species on the farm was 7 compared to 6 in the Nature Reserve. Fence line 18 number of plant species 16 Nature reserve Farm 14 12 10 8 6 4 2 0 0 1 2 3 4 5 6 7 8 9 10 11 12 vegetation surveys Figure 19: Comparison between the number of plant species on both sides of a fence between Goegap Nature Reserve and a farm. I made 11 pairs of vegetation surveys on the same level. T=2.5; p = 0.01 There were too few small mammals trapped for statistical comparisons. On the Farm one G. paeba and three A. namaquensis were trapped. In the nature reserve four R. pumilio and one G. paeba were trapped. Trapping data can be found in Table 6 (Appendix). Discussion 39 5. Discussion Conservation (i.e. the maintenance and protection of natural habitats) is one of the main challenges and duties of our time, as it also functions to protect our own endangered habitat. An important part of conservation is the protection of species. All species in an ecosystem are connected to each other in a more or less direct way (Begon et al. 1998). The extinction of one species often has severe consequences for other species in its ecosystem (Begon et al. 1998). Humans are also involved given the fact that earth itself is the biggest known ecosystem. Biodiversity is also of immense value with regards to adaption. No matter if from human interference or more natural caused, our environment will change during the following decades. With a more variable genpool it will be much easier for life to adapt to these changes. Given the current rate of extinction and the explosion of human population density, conservation is of increasing importance. In places where many species live in a small area, conservation is most effective (Myers et al. 2000). One of these places is the Succulent Karoo with its extraordinary floral diversity (Myers et al. 2000). To provide effective conservation methods for plant diversity, it is essential to find out which factors influence or maintain biodiversity. Herbivore animals feeding on plants might be one of these factors. In the Succulent Karoo small mammals are of special importance, because they have high population densities here and larger herbivores are relatively rare. The influence of small mammals on plant diversity was investigated in this study for the first time. 5.1. Correlation between small mammals and plants Previous studies on the influence of predators on biodiversity revealed contradictory results with predators either increasing (Paine 1966, Lubchenco 1978) or decreasing (Harper 1969; Lubchenco 1978) the diversity of their foodplants. This difference might be due to whether the preferred prey species belongs to the dominant or subdominant species in each case. It is crucial to know about this because predators are only assumed to increase the diversity of their prey if they prefer dominant prey species, making space for subdominant species, which would otherwise be outcompeted (Lubchenco 1978). This predation-hypothesis was first described by Paine (1966) and has so far been tested only in habitats Discussion 40 with average biodiversity. To my knowledge my study is the first to test the predation hypothesis on a biodiversity hotspot: the Succulent Karoo (Myers et al. 2000). I found that plant diversity correlated significantly with the number of trapped small mammals and the number of their species in winter (July/August). This was true for the total number of plant species and for the perennial plant species separately. In summer (November/December) only the association between perennial plant species and the number of small mammal species showed a significant, positive correlation. A correlation between perennial plant species and the number of trapped small mammals however could not be demonstrated. These results support the predation-hypothesis and indicate that small mammals could increase biodiversity in the Succulent Karoo. However I cannot be sure in this stadium, whether the relation I found was due to an influence of small mammals on plants or the other way around. Maybe small mammals have a positive influence on plant diversity, but not from reducing dominant plant species. Indirect ways of influence are possible. Small mammals digging burrows might for example increase plant diversity, because their burrows store water during rainfall and keep it available for plants. However, only three of the 10 trapped small mammal species burrowed (the three gerbil species). Furthermore it is possible that other factors, such as altitude and edaphic factors (see 3.3.4, 3.3.5 and 3.3.6) influenced plants and small mammals in a similar way, although this is highly unlikely, because of the results the general linear model revealed. In winter plant biodiversity correlated more strongly with the number of small mammal species than with the total number of small mammals, and in summer the only significant correlation I found was also with to the number of small mammal species and not with the total number of trapped individuals. The stronger influence of small mammal species on plant diversity is probably due to the range of their diet. One small mammal species alone affects possibly only a few plant species that it prefers as food-plants. Many small mammal species together, however, are likely to have a more variable diet and therefore a stronger influence. Additionally different species feed on different parts of their food plants (e.g. seeds, leaves, roots). Therefore a plant species eaten by only one small mammal Discussion 41 species might not be influences severely because the damage is done to only one part of the plant. Many small mammal species together however might have a much stronger influence, even if they have a low population density. One reason for the stronger correlation between small mammals and plants in winter might be the smaller cruising radius small mammals typically have in winter (Schubert 2005). The larger the cruising radius of herbivores is, the smaller should be the effect on plants in their surrounding, because the influence of a single individual is spread over a larger area. It is also possible that small mammals choose their habitat based on the diversity of their food plants, which is why the correlation between small mammals and plant diversity would be stronger in winter, when food is a more limited factor than in the early summer. The Succulent Karoo is a seasonal environment with abundant plant growth by ephemerals in spring, which is also the breeding season of most small mammals (Schradin & Pillay 2005; Jackson 1999). In my study as well I found both a higher abundance of small mammals and annual plant species at the beginning of summer. The correlation between small mammals and annual plant species could not be calculated for the winter-trapping-season season because annuals were nearly absent during this season (Cowling 1999). Even in summer when there were numerous annuals there was no significant correlation. Obviously small mammals and annuals have no remarkable influence on each other. Possibly the short time-span in which the two can interact plays a part here. Most of the annual plant species in Namaqualand are ephemeral and have a lifespan of only a few months. Small mammals on the one hand do not have enough time to reduce certain dominant or subdominant plant species significantly because annuals occur in great numbers for a relatively short period in spring and vanish quickly when it gets dry in summer. On the other hand, due to their short lifespan, annuals are not important for the over-winter survival of small mammals. Annuals are essential for their breeding season, but small mammals cannot rely on annuals over the whole year. This reduces the influence of annual plant species on the species composition of small mammals. In the future it would be good include other small mammal species that were not sampled in my study. With different types of traps for example it would be possible to trap small mammal species that do not enter the Shearman traps I used. Whistling rats (Parotomys brantsii, Parotomys littledalei) and subterranean Discussion 42 species like mole rats (Bathyergus janetta, Cryptomys hottentotus) belong to this group. Dassie rats (Petromus typicus) are probably not attracted by the bait I used. Another aspect that has not been taken into account in my studies is that insects certainly also are important herbivores (Zeller 2002) and to my knowledge there are no studies on their influence on biodiversity in Namaqualand or anywhere else. Another constraint of this study could be the unevenly distribution of the ten areas within the nature reserve. For methodological reasons the distances between the areas are very different (Fig. 1). The areas were chosen in order to be ecologically different, but nevertheless it cannot be excluded that the data from some areas are more similar to each other than others, because of the varying distance between them. The correlations I found support the hypothesis that small mammals affect floral diversity. However it cannot be said at the moment if there is a causal connection between small mammals and plants. A clear statement would require further investigations using an experimental approach. There are several fenced monitoring plots in Goegap Nature Reserve. The fence protects the plants growing inside the plots from bigger herbivores but not from small mammals. To prove the influence of small mammals on plant diversity it would be useful to fence these plots in a way that also excludes small mammals. A comparison between these fenced plots and adjacent unprotected areas over several years and during different seasons would make it possible to test the influence of small mammals on plant biodiversity experimentally. 5.2. Food-preference-test If small mammals influence plant biodiversity in the way predicted by the predation-hypothesis it would be expected that at least some of them prefer dominant plant species. Dominant plant species are not seriously threatened even if they are the preferred food-plants. Being eaten just prevents them from displacing subdominant plant-species. A preference for subdominant food-plants however could lead to their extinction. Discussion 43 5.2.1. Pilot study The pilot study revealed that R. pumilio preferred the two plant species Lessertia. diffusa and Lycium. cinereum. Lessertia. diffusa is an annual plant species and therefore just available for the mice in spring and early summer. This is also true for L. cinereum. Although this is a perennial shrub it foliates only in spring. R. pumilio seems to prefer this fresh food in general. The same seems to apply to O. unisulcatus. Nine out of the ten animals I tested preferred Mesembryanthemum guerichianum, which was the only annual plant species in the test. This can be easily explained by the higher nutrient value of annuals in comparison with perennials (Oftedal 2002). To exclude the preference for fresh plant material, only perennial shrubs, which are available throughout the year, were used in the second set of tests. 5.2.2. Second set of tests The influence of herbivores on their food-plant’s diversity depends decisively on the preference of herbivores for dominant or subdominant plants (Lubchenco 1978). In fact both of the tested species, O. unisulcatus and R. pumilio, showed a significant preference. Interestingly the two preferences did not point in the same direction. Whereas R. pumilio preferred the dominant food-plants in the tests, O. unisulcatus favoured the subdominant plant species. This result implies a positive influence from R. pumilio on plant diversity and a negative one from O. unisulcatus as explained above (see 5.1.). The choice of the tested plant species was done randomly. So it can be that some species were chosen that are not included in the animals’ diet or are even unpalatable. To be unpalatable is an effective strategy for plants to protect themselves from being eaten. This way subdominant or formerly subdominant plants can resist their palatable competitors that would probably outcompete them otherwise. This can be seen most clearly on rangeland, where plants that are unpalatable for livestock can spread unchecked. One of these plants is the shrub Z. retrofractum, which is unpalatable for livestock, but the preferred food-plant of R. pumilio. Z. retrofractum leaves contain toxic substances in their sap but not all leaves are toxic to the same extend (pers. commun. du Toit, state botanist, Northern Cape Province). R. pumilio might be able to feed specifically only on the non-toxic very small leaves and seeds (size of a pinhead), which bigger ungulates Discussion 44 like goats and sheep are unable to do. Livestock cannot choose the few palatable leaves out of the large number of leaves growing on one branch. Z. retrofractum is a dominant plant species and its population density can only be regulated by small mammals. This can be of great interest for the farmers in Namaqualand, who prefer to have palatable plants on their land, but support Z. retrofractum without meaning to by high stocking rates, i.e. reducing its palatable competitors. Small mammals are often seen as pests and their profitable side is overlooked. There is certainly a need for food-preference-tests with other herbivore species and it would be beneficial to repeat them with more plant species on R. pumilio and O. unisulcatus. This would lead to a more general understanding of the influence herbivores have on their food-plants in the Succulent Karoo. 5.3. Plant biodiversity around bush-Karoo rat nests To measure a possible effect of small mammals on biodiversity directly, the plant diversity around occupied/unoccupied nests of O. unisulcatus, was determined. O. unisulcatus is a central place forager and therefore especially qualified for this study. They feed in the direct surroundings of their nests so that an influence of a rat’s presence can be determined by investigating the area around an occupied nest. The population density of O. unisulcatus was not as high as usual, because a drought during the previous year made them locally extinct on the study site, which they only started to colonise again (from a neighbouring farm) in 2004, when this study took place. Therefore there were occupied and abandoned areas, which could be compared to each other. These circumstances were for example not true for R. pumilio, which have a different foraging strategy and roam their homerange searching for food. A study on R. pumilio was also made impossible by the fact that it builds its nest in bushes in such a way that one cannot see them from outside. Although several nesting sites were known from other studies, a study was not possible because in the concerning area the population density was so high that it was impossible to find unoccupied areas for comparison. While the results of the food-preference-tests indicated that O. unisulcatus might have a negative influence on plant diversity, the vegetation surveys around the nests suggested the opposite. As the study was designed to have occupancy as the only difference between the compared nests, the higher diversity around Discussion 45 occupied nests could be due either to rats increasing plant biodiversity or rats choosing nesting sites with higher plant biodiversity. I cannot say which possibility is correct with this correlative study. Additionally, there might be other factors, unrecognised by me, influencing the presence of rats and plant diversity in the same way. Should the rats choose their nesting site according to floral diversity, the duration of occupancy in the moment of investigation might affect the result. Since the food-preference-tests implied a negative influence of O. unisulcatus, it is possible that a recently occupied nest has a high plant diversity in its surroundings, because the animals have chosen the nesting site according to plant diversity. The surroundings of a nest that has been exposed to the potentially negative influence of O. unisulcatus for a long time could have reduced plant diversity. It is known, however, that many nests are used for several years (Brown & Willan 1991; Schradin unpublished data). Therefore, it is not likely that O. unisulcatus do lasting damage to their environment by, for example, causing the extinction of subdominant plant species because they would not be able to live in the same region for years in that case. The high plant diversity around occupied nests of O. unisulcatus can also be reconciled with the results of the food-preference tests in another way. It is possible that other small mammals, which in contrast to O. unisulcatus prefer dominant food-plants, but also prefer to stay next to O. unisulcatus, compensate for the negative influence of O. unisulcatus. R. pumilio for example use unoccupied nests of O. unisulcatus (Schradin in press). In this case I measured not the direct effect of O. unisulcatus on the surroundings of their nests, but the influence of other small mammals that are associates with O. unisulcatus. 5.4. Other factors that might influence plant biodiversity The pH-values of the soil samples from the ten areas showed little variation. Only the areas 9 and 10 had remarkably low pH-values. These two areas were at a much higher altitude compared to the others and are also most distant from them, which is a probable explanation for this deviation. Areas 9 and 10 also have high percentages of organic particles in the soil that are known to decrease pHvalue because of their humic substances (Gisi 1997). The highest percentage of organic components in the soil was found in areas 1, 5, 9 and 10. Area 10 consists mainly of big rocks, so the soil samples Discussion 46 were taken from the sandy patches in between. Probably organic soil particles are concentrated in these places. The same could be true for the areas 1 and 9, which are both situated on the foot of a hill, where wind and rain might deposit organic particles. Area 5 did not only have a high percentage of organic soil components, but also high concentrations of different metal ions. The area is situated next to an old cooper mine. Strong winds, which are quite common in Namaqualand, often blew grey dust from the mining dump to area 5. Most probably this is the reason for the high concentrations of metal ions. The high concentration of organic particles however remains surprising. In spite of high nutrient concentrations and the organic particles in the soil, there was no extraordinarily high plant diversity or plant cover in area 5. The sparse vegetation in areas 3, 4 and 6 can explain the low percentage of organic components there. The Cluster-analysis that included additional measured factors showed clearly that small mammals and plants are more abundant in areas 8, 9 and 10 (cf. Tab. 2). These areas are also at the highest average altitude and have the highest average percentage of organic particles in the soil. The plant diversity might directly affect the organic soil particles and the small mammal number and species number, or the other way around. The altitude, however, is independent from plant diversity. It is surprising on the first view that biodiversity seems to increase with increasing altitude, because in the Andean forests the opposite effect can be found (Gentry 1988). On the second view one can see that areas 8, 9, and 10 also have moderate or plenty rainfall (cf. Tab. 1). Higher areas seem to have a tendency to get more rainfall, which would explain the high plant diversity. Clouds coming from the Atlantic rain out on the higher slopes whereas they often just pass by in lower regions. For this reason the Kamiesberg area, the bioregion with the highest altitude in Namaqualand, has got the highest average annual rainfall of all bioregions here (Cowling 1999). The general linear model showed that plant diversity could be explained by small mammal diversity in winter and in summer. Concerning this result I can now exclude the alternative explanation that other factors influenced plants and small mammals in the same way and caused the correlation I found. Combining this result with the others I can say now that small mammals indeed affect plant diversity in Namaqualand. It was, however, surprising that plant diversity in winter can also be explained by the concentration of manganese in the soil. Manganese Discussion 47 is an essential nutrient for plants, but this is also true for other nutrients. It is even more surprising that this connection between manganese and plant diversity was only found in winter. So the importance of manganese seems to vary within the year. 5.5. Fence line The comparison between the floras of a Nature Reserve with those of a neighbouring farm showed a significantly higher diversity on the farm. The plants of the farm have been exposed to the influence of goats, sheep and cattle for many years. If the stocking had a positive influence on the diversity of plants, this could be due to livestock preferring dominant plant species and thereby keeping them away from outcompeting subdominant food-plants. Because herbivores decrease the diversity of their food-plants if they prefer subdominant plant species (Lubchenco 1978) and the farm had a higher plant diversity in comparison to the ungrazed nature reserve, it is unlikely that there is a preference for subdominant plants. The edibility of the plants was however unaccounted for in this study. Possibly many of the subdominant plant species are unpalatable and therefore not eaten by ungulates. This possibility requires further investigation. A high reproduction of unpalatable plants (e.g. Galenia africana) was found in several studies (Todd & Hoffman 1999). Furthermore, one can think of other ways for livestock to increase biodiversity. Their dung for example might be an advantage for plants growing on farmland. The investigated farm has not been grazed for nearly 2 years and thus might be in a phase of regeneration in which many pioneer-plants increase the population density. The season in which the study was conducted might also be of importance. The vegetation surveys were done in spring and many annual plant species were included in the investigation. Grazing has a particularly negative influence on different shrubs, which were reduced by livestock. Unpalatable plants, like Z. retrofractum and G. africana are left behind with sandy patches between them. On these sandy patches a great number of annuals can be found in spring. Because the investigated farm was not grazed in 2004, annual plant species could easily Discussion 48 establish themselves. Probably the study would have had another result if it had been conducted in winter. Besides stock farming, other factors might be responsible for the species richness on the farm. The farmers planted some trees for example and the areas on the two sides of the fence differed in heterogeneity. The study area on the farm was much more heterogeneous. Although there was a plain area and a sandy and rocky area on both sides, there also was a waterhole on the farm. Water is of enormous importance for plants, especially in arid regions. So it is likely that this water hole had a positive effect on the plants of the farm. For methodological reasons it was not possible to conduct this study in a more homogeneous area. It would be important to repeat this study in a more appropriate area. This would also be interesting with regards to small mammals. Joubert and Ryan (1999) mentioned that the species composition on high stocking rate rangeland is a subset of the species occurring in areas with lower stocking rates. In my study there was neither a remarkable difference in small mammal species number nor in number of trapped individuals. Only species composition differed. G. paeba was trapped on the farm and on the nature reserve. This species is one of the most abundant ones in the Hardeveld (this study) and seems to be very tolerant regarding its habitat. As G. paeba is nocturnal (Stuart & Stuart 2001) and lives in burrows, one can imagine that it is not so dependent on plant cover because there is no need to hide from birds of prey, which are mainly diurnal and no shrubs are required as nesting sites. G. paeba was also trapped on highly overgrazed rangeland in other studies (Joubert & Ryan 1999). It does not seem to be a good indicator species. A. namaquensis was also trapped on the farm. This species is highly dependent on rocky areas (Stuart & Stuart 2001), but was trapped here near the reeds at the mentioned water hole. Next to this place is a heap of stone wreckage that might have been used by the mice as substitute for rock crevices. R. pumilio was only trapped in the Nature Reserve, which had considerably more shrubs providing cover and nesting sites. When trapping for food choice tests on the farm and investigating bushKaroo rat nests, I also trapped several O. unisulcatus, a few R. pumilio and one Mus minutoides on the farm right next to my transect. Additionally it is known from earlier studies that O. unisulcatus and Macroscelides proboscideus live in the nature reserve next to the fence (Schradin unpublished data). It is surprising that Discussion 49 these species were not trapped during the actual fence-line-study (four trapping days/nights). Possibly the trapping sites were not optimally chosen. Should this study be reproduced in another place, it is advisable to increase the number of trapping days. Although a clear statement about the effect of grazing on plant diversity cannot be given in the end, the study still has value if one considers it to be a pilot study for following investigations, which can hopefully be conducted under better circumstances. Conclusions 50 6. Conclusions The results of my studies, though being mainly correlative, indicate an influence of small mammals on plant diversity in the Succulent Karoo. This knowledge could make it easier in the future to protect this biodiversity-hotspot. Conservation is hardly possible without knowing the ecological background. From this study it is now clear that it is not enough to protect the threatened plant species directly. It is also necessary to consider small mammals in conservation programs, even if they are not threatened themselves. Additionally, further investigations are urgently needed, especially experimental ones that exclude small mammals from certain areas and allow a direct comparison between areas influenced and protected from small herbivorous mammals. References 51 7. References ANDREWS, P. & O`Brien, E. M. 2000. Climate, vegetation, and predictable gradients in mammal species richness in southern Africa. Journal of Zoology, 251, 205-231. BEGON, M. E., HARPER, J. L., TOWNSEND, C. R. 1998 Ökologie, Heidelberg, Berlin: Spektrum Akademischer Verlag BROOKS, PM 1982. Aspects of the reproduction, groth and development of the four-striped mouse, Rhabdomys pumilio (Sparrman, 1784). Mammalia, 46, 53-64 BROWN, E., WILLAN, K. 1991. Microhabitat selection and use by the bush Karoo rat Otomys unisulcatus in the Eastern Cape Province. South African Journal of Wildlife Reserves, 21 (3), 69-75 CORBET, G. B. 6 J. HANKS. 1968. A revision of the elephant-shrews, Family Macroscelididae. Bulletin of the British Museum (Natural History), Zoology 16:47111. COWLING, R. M., Esler, J. J. & Rundel, P. W. 1999. Namaqualand, South Africa an overview of a unique winter-rainfall desert ecosystem. Plant Ecology, 142, 321. COWLING, R. M. 1999. Reserve selection in the Succulent Karoo, South Africa: coping with high compositional turnover. Plant Ecology, 142, 35-55. CURTIS, B. A. & PERRIN, M. R. 1979. Food preferences of the vlei rat (Otomys irroratus) and the four-striped mouse (Rhabdomys pumilio). South African Journal of Zoology, 14, 224-229 DARWIN, C. 1859. On the Origin of the Species by Means of Natural Selections, or the Preservation of the Favoured Races in the Struggle for Life. London (Murray J.) References 52 DAVIS, R. M. 1974. The distribution of some southern African mammals (Mammalia: Insectivora, Rodentia), Annals of the Transvaal Museum., 29, 135-184 DESMET, P. G. & COWLING, R. M. 1999. The climate of the Karoo – a functional approach. In: Dean, W. R. J. & Milton, S.J. (eds), The Karoo: Ecological Patterns and Processes. Cambridge: Cambridge University Press, DEWSBURY, DA & DAWSON; WW, 1979. African four-striped grass mouse (Rhabdomys pumilio), a diurnal-crepuscular muroid rodent, in the behavioural laboratory. BEHAVIOR RESEARCH METHODS INSTRUMENTS & COMPUTERS, 11, 329-333 DIEKMANN, R. C. 1979. Note on the smaller mammals of the Hester Malan Nature Reserve, Springbok, Namaqualand. South African Journal of Zoology 14, 85-89 Du PLESSIS, A. & KERLEY, G. I. H. 1991. Refuge strategies and habitat segregation in two sympatric rodents Otomys unisulcatus and Parotomys brantsii. Journal of Zoology London, 224, 1-10 Du PLESSIS, A., ERASMUS, T. & KERLEY, G. I. H. 1989. Thermoregulatory patterns in two sympatric rodents Otomys unisulcatus and Parotomys brantsii. Comparative Biochemical Physiology. 94, 215-220 GELDEBLOM, C. M. & Bronner, G. N. 1995. Patterns of distribution and protection status of the endemic mammals in South Africa. South African Journal of Zoology, 30, 127-135. GENTRY, R. V. 1988. Creation's Tiny Mystery. 2nd edit., Knoxville: Earth Science Associates GISI, U. 1997. Bodenökologie, Stuttgart; New York: Thieme Verlag References 53 HARPER, J. L. 1969 The role of predation in vegetational diversity. Brookhaven Symposium in Biology. 22, 48-62 HILTON-TAYLOR, C. 1996. Patterns and characteristics of the flora of the Succulent Karoo Biome, southern Africa. In: van der Maesen, L. J. E., van der Burgt, X. M. & van Medenbach de Rooy, J. M. (eds), The Biodiversity of African Plants. 58-72, Dordrecht, The Netherlands: Kluwer Academic Pulishers HILTON-TAYLOR, C. & LEROUX, A. 1989. Conservation status of the Fynbos and Karoo biomes. In: Huntley, B. J. (ed), Biotic diversity in Southern Africa: concepts and conservation. 202-223. Cape Town: Oxford University Press IHLENFELDT, H. D. 1994. Diversifications in an arid world: The Mesembryanthemaceae. Annual Review of Ecology and Systematics, 25, 521-546 JACKSON,T. P. 1999. The social organization and breeding system of Brants´ whistling rat (Parotomys brantsii). Journal of Zoology London, 247, 323-331. JOUBERT, D. F. & RYAN, P. G. 1999. Differences in mammal and bird assemblages between commercial and communal rangelands in the Succulent Karoo, South Africa. Journal of Arid Environments, 43, 287-299. JÜRGENS, N., GOTZMANN, I. H. & COWLING, R. M. 1999. Remarkable mediumterm dynamics of leaf succulent Mesembryanthemaceae shrubs in the winterrainfall desert of northwestern Namaqualand, South Africa. Plant Ecology, 142, 8796. KERLEY, G. I. H. 1992b. Trophic status of small mammals in the semi-arid Karoo, South Africa. Journal of Zoology London, 226, 563-572. KINGDON, J. 1974. East African Mammals Vol. 1, Chicago: The University of Chicago Press References 54 LAMPRECHT, J. 1999. Biologische Forschung von der Planung bis zur Publikation. Fürth: Filander Verlag LECHEMERE-OERTEL, R. G. & COWLING, R. M. 2001. Abiotic determinants of the fynbos / succulent karoo boundary, South Africa. Journal of Vegetation Science 12: 75-80 LEROUX, A., SCHELPE, T.& WAHL, Z. 1997. South African Wild Flower Guide 1: Namaqualand. Cape Town: Botanical Society of South Africa LOMBARD, A. T., HILTON-TAYLOR, C., REBELO, A. G., PRESSEY, R. L. & COWLING, R. M. 1999. Reserve selection in the Succulent Karoo, South Africa: coping with high compositional turnover. Plant Ecology, 142, 35-55 LUBCHENCO, J. 1978. Plant species diversity in a marine intertidal community: Importance of herbivore food preference and algal competitive abilities. The American Naturalist, 112, 23-39 MANNING, J. & GOLDBLATT, P. 1998 South African Wild Flower Guide 7: West Coast. Cape Town: Botanical Society of South Africa MILTON, S. J., DEAN; W. R. J., DUPLESSIS; M. A. & SIEGFRIED; W. R. 1994. A conceptual model of arid rangelands degradation. Bioscience, 44, 70-76 MILTON, S. J., YEATON, R. I., DEAN, W. R. J. & VLOK, J. H. J. 1997. Succulent Karoo. In: Cowling, R. M., Richardson, D.M & Pierce, S.M. (eds.), The Vegetation of Southern Africa. 131.166. Cambridge: Cambridge University Press MURPHY, W.J., ELZIRIK, E., JOHNSON, W.E., ZHANG, YA-PHING, RYDER, O.A. & O’BRIEN, S.J. 2001. Molecular phylogenetics and the origin of placental mammals. Nature 409, 614-618. References 55 MYERS, N., MITTERMEIER, R. A., MITTERMEIER, C. G., FONSECA, G. A. B. D. & KENT, J. 2000. Biodiversity hotspots for conservation priorities. Nature, 403, 853-858. NOWAK, R. M. (ed.). 1991. Walker's Mammals of the World (Fifth Edition). Baltimore: The Johns Hopkins University Press. OFTEDAL, O.T. 2002. Nutritional ecology of the desert tortoise in the Mohave and Sonoran deserts. In Van Devender, T.R. (ed) The Sonoran Desert Tortoise. Natural History, Biology, and Conservation. 194-241. Tuscon: University of Arizona Press PAINE, R.T. 1966. Food web complexity and species diversity. The American Naturalist, 100, 65-75. PILLAY, N. 2001. Reproduction and postnatal development in the bush Karoo rat Otomys unisulcatus (Muridae, Otomyinae). Journal of Zoology London, 254, 515- 520 RATHBUN, G.B. & FONS, R. 1988. Russelspringer. In: B. Grzimek (ed.). Grzimeks Enzyklopadie Saugetiere. Pp. 520-531, München, W. Germany: Kindler Verlag,. RÖSCH, H. 2001. The identification and description of the management units of the Goegap Nature Reserve. Koedoe, 44(1), 17-30 ROSSA; B. & VON WILLERT, D. J. 1999. Physiological characteristics of geophytes in semi-arid Namaqualand. South Africa. Plant ecology, 142, 121-132 SCHRADIN, C. in press. Whole day follows of the striped mouse, Journal of Ethology References 56 SCHRADIN, C. in press. , Nest site competition in two diurnal rodents of the succulent karoo, Journal of Mammalogy SCHRADIN, C. & PILLAY, N. 2005. Demography of the striped mouse (Rhabdomys pumilio) in the succulent karoo. Mammalian Biology in press SCHUBERT, M. 2005. Female Reproductive Strategies in the Striped Mouse: Communal versus Singular Breeders. Unpublished Diploma thesis, University of Bayreuth, Germany. SHEARING, D. & HEERDEN, K. V. 1998. South African Wild Flower Guide 6: Karoo. Cape Town: Botanical Society of South Africa SHORTRIDGE, G. 1934. The mammals of South West Africa, Vol. 1. London: William Heinemann SKINNER, J. D. & SMITHERS, R. H. N. 1990. The mammals of the Southern African Subregions 2nd edn. Pretoria: University of Pretoria STUART, C. & STUART, T. 2001. A Field Guide to Mammals of Southern Africa. Chelsea Green Publishing Co, U.S. TODD, S. W. & HOFFMAN, M. T. 1999. A fence-line contrast reveals effects of heavy grazing on plant diversity and community composition in Namaqualand, South Africa. Plant Ecology, 142, 169-178. TODD, S. W. & HOFFMAN, M. T. 1999. Correlates of stocking rate and overgrazing in the Leliefontein Communal Reserve, central Namaqualand. African Journal of Range & Forage Science, 17, 36-45 VAN JAARSVELD, E. 1987. The riches of South Africa and Namibia, Aloe, 24, 4592 References 57 VAN ROOYEN, G., STEYN, H.P. & De VILLIERSSOUTH, R. 1999. African Wild Flower Guide 10: Cederberg – Clanwilliam and Biedouw Valley. Cape Town: Botanical Society of South Africa WEIß, J., MAEß, J., NEBENDAHL, K., ROSSBACH, W. (eds.) 1995. Haus- und Versuchstierpflege, Stuttgart, Jena, New York : Gustav Fischer Verlag WILMANNS, O. 1989. Ökologische Pflanzensoziologie, 4., überarb. Aufl., Heidelberg Wiesbaden ZELLER, U. 2002. Functional Zoodiversity in Southern Africa under changing environments and human use. Unpublished data Appendix 58 8. Appendix Table 4: Trapping data from the winter-trapping season species number date 1 6.7.2004 M. minutoides 2 8.7.2004 M. minutoides 0 6.7.2004 A. namquensis 1 5.7.2004 A. namquensis 1 8.7.2004 A. namquensis 1 8.7.2004 E. rupestris 1 5.7.2004 G. paeba 2 5.7.2004 G. paeba 3 6.7.2004 G. paeba 4 9.7.2004 G. paeba 1 5.7.2004 M. proboscideus 2 5.7.2004 M. proboscideus 6 10.7.2004 G. paeba 7 11.7.2004 G. paeba 1 10.7.2004 R. pumilio 1 10.7.2004 R. pumilio 1 11.7.2004 R. pumilio 1 14.7.2004 R. pumilio 1 10.7.2004 G. valinus 1 15.7.2004 G. paeba 2 19.7.2004 G. paeba 1 20.7.2004 G. paeba 2 20.7.2004 G. paeba 2 21.7.2004 G. paeba 3 20.7.2004 G. paeba 4 20.7.2004 G. paeba 5 20.7.2004 G. paeba 12 21.7.2004 G. paeba 17 23.7.2004 G. paeba 18 23.7.2004 G. paeba 20 28.7.2004 G. paeba 1 21.7.2001 R. pumilio 1 21.7.2004 R. pumilio 1 28.7.2004 R. pumilio 1 28.7.2004 R. pumilio 2 23.7.2004 R. pumilio 1 20.7.2004 O. unisulcatus 6 20.7.2004 G. paeba 7 20.7.2004 G. paeba 8 20.7.2004 G. paeba 9 21.7.2004 G. paeba 10 21.7.2004 G. paeba 11 21.7.2004 G. paeba 13 21.7.2004 G. paeba 14 21.7.2004 G. paeba 15 21.7.2004 G. paeba time area station weight (g) sex 20:05 1 30 08:06 1 28 19:55 1 15 19:55 1 29 m 06:31 1 29 m 07:53 1 7m 21:20 2 14 21:30 2 29 20:22 2 10 06:45 2 4 27 20:20 2 28 f 20:25 2 30 f 21:28 4 27 23 19:42 4 29 24 19:45 4 11 32 21:12 4 11 21:12 4 9 37 07:00 4 7 30 21:13 4 15 26 m 21:10 5 19 29 06:25 5 5 26 21:00 7 4 25 21:05 7 10 27 19:20 7 4 27 21:10 7 12 26 m 21:17 7 24 27 m 21:17 7 27 30 m 20:50 7 5 24 f 06:20 7 19 27 06:30 7 30 27 m 06:30 7 1 25 16:43 8 3 52 m 18:10 8 3 m 09:35 8 2 50 m 11:10 8 2 m 11:00 8 1 37 m 18:12 8 2 106 m 21:45 8 13 27 21:50 8 19 25 f 22:02 8 24 25 f 19:40 8 26 30 m 19:47 8 9 30 20:00 8 2 26 21:15 8 30 25 f 21:25 8 19 28 21:30 8 13 25 - - = no data available m = male f = female X =animal escaped before it could be marked Recapture Appendix species number date time area station weight (g) sex 16 21.7.2004 21:36 8 5 28 G. paeba 16 23.7.2004 06:50 8 24 25 G. paeba 19 23.7.2004 06:59 8 21 26 G. paeba 1 20.7.2004 21:55 8 24 23 f M. proboscideus 2 21.7.2004 20:03 8 1 49 f M. proboscideus 3 28.7.2004 06:50 8 11 40 f M. proboscideus 9 29 55 m 1 4.8.2004 10:45 G. paeba 1 31.7.2004 19:43 9 8 27 m G. paeba 1 4.8.2004 06:12 9 4 24 m G. paeba 2 31.7.2004 21:05 9 29 20 f G. paeba 3 3.8.2004 06:09 9 5 28 m G. paeba 3 4.8.2004 06:20 9 6 27 m G. paeba 9 4 25 4 3.8.2004 07:46 G. paeba 5 4.8.2004 06:12 9 3 26 G. paeba 9 8 25 m 6 4.8.2004 06:26 G. paeba 7 4.8.2004 06:37 9 25 22 G. paeba 3 31.7.2004 18:02 9 26 55 E. edwardii 4 31.7.2004 18:10 9 30 54 f E. edwardii 4 4.8.2004 07:55 9 28 53 E. edwardii 9 5 41 m 1 29.7.2004 19:30 M. proboscideus 1 31.7.2004 19:38 9 5 40 m M. proboscideus 1 3.8.2004 06:14 9 9 45 m M. proboscideus 1 4.8.2004 06:31 9 13 46 m M. proboscideus 1 4.8.2004 07:50 9 2 43 M. proboscideus 2 29.7.2017 19:37 9 12 54 f M. proboscideus 2 31.7.2004 19:34 9 2 45 f M. proboscideus 2 3.8.2004 07:54 9 13 57 f M. proboscideus 1 29.7.2004 19:56 10 3 10 m M. minutoides 2 31.7.2004 20:06 10 3 10 M. minutoides 0 4.8.2004 07:04 10 28 12 M. minutoides 1 29.7.2004 18:31 10 28 f A. namaquensis 1 31.7.2004 18:35 10 28 47 f A. namaquensis 1 31.7.2004 20:42 10 28 46 f A. namaquensis 2 29.7.2004 18:40 10 29 39 f A. namaquensis 2 29.7.2004 20:27 10 26 44 f A. namaquensis 2 31.7.2004 18:36 10 29 38 f A. namaquensis 2 3.8.2004 06:43 10 27 40 A. namaquensis 2 4.8.2004 07:04 10 29 43 f A. namaquensis 2 4.8.2004 08:22 10 27 46 f A. namaquensis 3 29.7.2004 18:40 10 29 39 m A. namaquensis 3 29.7.2004 20:40 10 29 50 m A. namaquensis 3 31.7.2004 18:36 10 30 47 m A. namaquensis 3 31.7.2004 20:46 10 29 46 m A. namaquensis 3 31.7.2004 21:22 10 23 44 m A. namaquensis 3 3.8.2004 08:37 10 28 46 m A. namaquensis 4 29.7.2004 20:10 10 17 52 m A. namaquensis 4 3.8.2004 08:06 10 1 58 m A. namaquensis 5 29.7.2004 20:27 10 28 57 m A. namaquensis 5 31.7.2004 20:20 10 11 51 m A. namaquensis 6 29.7.2004 21:35 10 28 46 A. namaquensis 59 - = no data available m = male f = female X =animal escaped before it could be marked Recapture Appendix species A. namaquensis A. namaquensis A. namaquensis A. namaquensis A. namaquensis E. edwardii E. edwardii E. edwardii E. edwardii E. rupestris E. rupestris E. rupestris E. rupestris E. rupestris E. rupestris 60 number date 6 31.7.2001 7 31.7.2004 7 3.8.2004 7 4.8.2004 X 29.7.2004 1 3.8.2004 1 4.8.2004 2 29.7.2004 2 31.7.2004 1 29.7.2004 1 3.8.2004 1 4.8.2004 2 31.7.2004 2 3.8.2004 0 4.8.2004 time area station weight (g) sex 20:34 10 25 39 20:25 10 17 46 f 08:16 10 10 46 f 08:59 10 21 45 f 20:20 10 22 06:30 10 1 50 m 06:50 10 3 52 m 21:20 10 3 52 16:38 10 1 60 18:18 10 25 74 f 08:25 10 20 64 f 07:04 10 30 65 f 18:33 10 23 59 08:30 10 21 58 f 08:10 10 20 63 m Table 5: Trapping data from the summer-trapping season species A. namaquensis A. namaquensis A. namaquensis A. namaquensis E. rupestris E. rupestris E. rupestris E. rupestris E. rupestris E. rupestris E. rupestris E. rupestris E. rupestris E. rupestris E. rupestris E. rupestris E. rupestris E. rupestris E. rupestris E. rupestris E. rupestris R. pumilio R. pumilio R. pumilio G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba number date time area station weight (g) sex 1 10 30 0 8.11.2004 22:24 0 10.11.2004 04:40 1 13 33 20 7.11.2004 22:18 1 9 28 f 30 8.11.2004 22:19 1 9 57 0 7.11.2004 20:58 1 9 25 f 20 7.11.2004 17:48 1 9 61 m 20 10.11.2004 06:00 1 30 64 m 20 11.11.2004 07:36 1 12 65 30 7.11.2004 17:55 1 12 73 f 30 7.11.2004 20:55 1 730 8.11.2004 18:02 1 5 73 f 30 8.11.2004 19:07 1 730 8.11.2004 21:05 1 10 30 10.11.2004 07:30 1 5 78 30 11.11.2004 06:05 1 4 80 100 8.11.2004 18:03 1 10 28 f 100 8.11.2004 21:00 1 9 29 f 100 10.11.2004 06:01 1 9 30 100 11.11.2004 06:10 1 9 34 200 10.11.2004 04:37 1 10 31 f 200 11.11.2004 06:08 1 7 25,5 0 10.11.2004 07:50 2 30 44 m 0 11.11.2004 07:50 2 29 48 m 561 11.11.2004 09:00 2 30 48 m 0 11.11.2004 05:00 2 4 16,5 f 2 7.11.2004 22:41 2 19 30 m 2 11.11.2004 05:11 2 17 34 4 7.11.2004 22:44 2 21 33 m 4 8.11.2004 22:50 2 21 30 4 11.11.2004 05:05 2 7 34 10 11.11.2004 05:14 2 25 29 m - = no data available m = male f = female X =animal escaped before it could be marked Recapture Appendix species G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba R. pumilio O. unisulcatus O. unisulcatus G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba M. proboscideus G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba M. proboscideus M. proboscideus M. proboscideus M. proboscideus M. proboscideus M. proboscideus M. proboscideus M. proboscideus M. proboscideus M. proboscideus M. proboscideus M. proboscideus 61 number date time area station weight (g) sex 20 7.11.2004 22:36 2 11 16 m 2 30 25 f 30 7.11.2004 22:47 100 8.11.2004 22:42 2 7 29 200 8.11.2004 22:46 2 11 15 m 300 10.11.2004 04:54 2 11 18 m 300 11.11.2004 05:09 2 11 18 300 11.11.2004 06:00 2 11 2 30 29 X 10.11.2004 05:00 X 11.11.2004 05:17 2 26 28 3 18.10.2004 06:17 3 27 32 3 4 36 f 1 19.10.2004 04:39 4 5 113 m 1 15.10.2004 19:25 2 18.10.2004 06:32 4 5 91 f 4 19 8f 0 15.10.2004 21:00 1 15.10.2004 21:10 4 26 13 m X 18.10.2004 04:55 4 72 18.10.2004 04:58 4 9 23 2 19.10.2004 05:05 4 23 27 m 4 27 32 3 18.10.2004 06:17 7 19.10.2004 04:58 4 10 26 f 4 30 32 f 1 19.10.2004 05:10 5 15 14 0 2.11.2004 10:31 0 3.11.2004 10:28 5 11 14 f 20 2.11.2004 10:34 5 17 33 f 30 2.11.2004 09:10 5 5 16 m 30 6.11.2004 04:50 5 5 15 100 2.11.2004 10:39 5 23 28 f 100 5.11.2004 04:55 5 17 31 200 5.11.2004 04:48 5 3 15 m 300 5.11.2004 04:58 5 30 22 m 800 6.11.2004 03:17 5 17 31 m 10 6.11.2004 05:00 5 28 30 f 2 21.10.2004 22:14 7 25 33 2 22.10.2004 22:10 7 28 31 m 2 25.10.2004 06:10 7 3 30 m 6 24.10.2004 04:28 7 9 33 f 10 25.10.2004 04:31 7 7 30 f 11 25.10.2004 04:40 7 21 28 m 0 25.10.2004 06:20 7 17 37 m 1 21.10.2004 19:04 7 1 30 f 1 24.10.2004 04:20 7 4 32 f 1 25.10.2004 06:05 7 1 37 f 2 21.10.2004 20:55 7 19 51 f 2 24.10.2004 04:35 7 19 53 f 7 28 52 f 3 21.10.2004 21:00 3 24.10.2004 04:40 7 30 53 f 4 21.10.2004 21:00 7 29 41 m 6 21.10.2004 22:08 7 9 38 m 7 22.10.2004 20:45 7 4 38 m 7 24.10.2004 07:19 7 4 42 m - = no data available m = male f = female X =animal escaped before it could be marked Recapture Appendix species R. pumilio R. pumilio R. pumilio R. pumilio R. pumilio R. pumilio R. pumilio R. pumilio R. pumilio R. pumilio R. pumilio R. pumilio R. pumilio R. pumilio R. pumilio R. pumilio R. pumilio R. pumilio O. unisulcatus O. unisulcatus O. unisulcatus O. unisulcatus O. unisulcatus O. unisulcatus G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba M. proboscideus M. proboscideus M. minutoides R. pumilio R. pumilio R. pumilio R. pumilio R. pumilio R. pumilio R. pumilio R. pumilio R. pumilio R. pumilio R. pumilio 62 number date time area station weight (g) sex 0 22.10.1004 17:54 8 11 55 m 0 24.10.2004 09:17 8 2 52 m 0 25.10.2004 07:55 8 12 50 m 1 21.10.2004 17:56 8 30 46 m 2 21.10.2004 19:20 8 29 44 f 2 22.10.2004 17:50 8 30 41 f 2 22.10.2004 19:15 8 30 41 2 24.10.2004 07:36 8 28 35 f 2 25.10.2004 07:50 8 29 41 3 24.10.2004 07:52 8 2 55 m 4 24.10.2004 09:17 8 4 60 f 8 1 59 5 25.10.2004 06:55 6 25.10.2004 06:55 8 2 60 f 8 11 59 m 7 25.10.2004 09:20 8 25.10.2004 09:25 8 29 41 f 9 25.10.2004 09:25 8 29 18 f 10 25.10.2004 09:13 8 5 15 m 11 24.10.2004 09:16 8 27 14 8 15 100 m 0 25.10.2004 06:37 1 22.10.2004 18:01 8 2 114 m 2 22.10.2004 21:12 8 2 121 m 3 24.10.2004 06:16 8 29 131 f 4 24.10.2004 07:45 8 10 102 5 25.10.2004 06:40 8 13 105 f 8 30 31 0 21.10.2004 22:30 0 24.10.2004 04:58 8 19 34 f 3 21.10.2004 22:38 8 4 29 f 4 22.10.2004 21:10 8 15 28 5 22.10.2004 22:28 8 27 25 f 5 21.10.2004 21:20 8 29 33 f 8 26 27 m 7 24.10.2004 04:54 7 25.10.2004 04:55 8 27 28 m 8 24.10.2004 05:02 8 17 30 m 8 25.10.2004 04:59 8 26 32 m 9 24.10.2004 05:08 8 2 28 f 12 25.10.2004 05:08 8 5 25 f 5 21.10.2004 21:20 8 29 33 f 8 25.10.2004 06:49 8 9 45 m 0 30.10.2004 05:03 9 28 18 X 26.10.2004 17:37 9 1 49 0 29.10.2004 07:47 9 29 juv. 1 26.10.2004 19:10 9 22 38 f 1 27.10.2004 17:40 9 26 37 f 1 29.10.2004 07:39 9 17 1 30.10.2004 09:00 9 17 34 10 27.10.2004 17:28 9 2 57 f 11 27.10.2004 17:30 9 5 24 m 12 27.10.2004 18:58 9 10 62 m 12 30.10.2004 07:18 9 10 62 13 27.10.2004 18:58 9 11 65 m - = no data available m = male f = female X =animal escaped before it could be marked Recapture Appendix species R. pumilio R. pumilio R. pumilio R. pumilio G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba G. paeba E. edwardii E. rupestris E. rupestris E. rupestris E. rupestris E. rupestris M. macroscelides M. macroscelides M. macroscelides M. macroscelides M. macroscelides M. macroscelides M. macroscelides M. macroscelides 63 number date time area station weight (g) sex 13 29.10.2004 07:44 9 23 13 29.10.2004 09:00 9 23 13 30.10.2004 07:25 9 23 59 13 30.10.2004 08:55 9 11 58 1 26.10.2004 20:42 9 17 13 1 27.10.2004 21:06 9 19 13 f 1 30.10.2004 04:48 9 15 15 9 23 13 2 26.10.2004 20:46 2 27.10.2004 21:16 9 28 15 m 2 29.10.2004 04:56 9 25 18 2 30.10.2004 05:00 9 27 17 f 9 25 13 m 3 26.10.2004 20:50 3 27.10.2004 21:14 9 26 16 3 29.10.2004 04:54 9 21 15 4 26.10.2004 22:02 9 3 11 m 4 27.10.2004 20:55 9 4 13 m 4 27.10.2004 22:00 9 3 11 4 29.10.2004 04:35 9 4 15 4 30.10.2004 06:04 9 3 16 5 26.10.2004 22:00 9 1 29 f 5 29.10.2004 04:29 9 1 30 5 30.10.2004 06:07 9 5 29 5 30.10.2004 04:32 9 5 30 f 6 26.10.2004 20:35 9 3 36 f 9 4 32 f 8 26.10.2004 22:06 8 27.10.2004 20:46 9 3 34 f 8 27.10.2004 22:02 9 78 29.10.2004 04:37 9 5 35 8 29.10.2004 05:59 9 5 36 8 29.10.2004 07:30 9 4 34 8 30.10.2004 04:39 9 7 37 9 26.10.2004 22:12 9 10 29 f 9 27.10.2004 21:00 9 15 30 f 9 29.10.2004 04:44 9 15 34 9 30.10.2004 04:45 9 12 34 f 20 30.10.2004 04:54 9 21 17 m 7 26.10.2004 19:20 9 28 77 f 4 27.10.2004 17:26 9 1 38 f 3 30.10.2004 04:28 9 1 67 m 3 30.10.2004 06:17 9 30 65 m 10 27.10.2004 20:45 9 1 79 f 0 29.10.2004 06:06 9 30 80 f 1 26.10.2004 19:05 9 7 45 f 1 29.10.2004 04:40 9 9 49 f 1 30.10.2004 04:31 9 4 47 f 1 30.10.2004 06:10 9 11 46 f 10 27.10.2004 21:10 9 21 18 m 10 29.10.2004 04:50 9 19 21 10 30.10.2004 04:52 9 19 22 f 11 29.10.2004 04:59 9 29 35 m - = no data available m = male f = female X =animal escaped before it could be marked Recapture Appendix species M. macroscelides M. macroscelides M. minutoides M. minutoides M. minutoides M. minutoides A. namaquensis A. namaquensis A. namaquensis A. namaquensis A. namaquensis A. namaquensis A. namaquensis A. namaquensis A. namaquensis A. namaquensis A. namaquensis A. namaquensis A. namaquensis A. namaquensis A. namaquensis A. namaquensis A. namaquensis E. rupestris E. rupestris E. edwardii E. edwardii E. edwardii E. edwardii 64 number date time area station weight (g) sex 12 29.10.2004 07:34 9 10 42 m 12 30.10.2004 04:41 9 11 37 m 25 91 27.10.2004 22:27 10 2 27.10.2004 22:29 10 27 9m 3 29.10.2004 05:36 10 27 13 m 70 30.10.2004 05:19 10 4 14 m 11 26.10.2004 22:28 10 1 29.10.2004 05:14 10 2 52 1 54 2 26.10.2004 20:59 10 2 27.10.2004 21:28 10 1 59 2 27.10.2004 22:17 10 12 29.10.2004 06:16 10 2 60 2 30.10.2004 05:14 10 1 56 f 6 26.10.2004 21:12 10 28 54 f 11 53 f 7 26.10.2004 19:34 10 7 27.10.2004 21:37 10 11 56 f 7 29.10.2004 05:25 10 17 57 7 26.10.2004 21:08 10 11 f 7 30.10.2004 05:35 10 21 57 30 24 f 10 27.10.2004 21:44 10 11 29.10.2004 05:19 10 11 33 f 7 28 m 20 30.10.2004 05:24 10 26 29 f 30 30.10.2004 05:40 10 3 26.10.2004 22:35 10 18 67 m 1 27.10.2004 17:55 10 21 63 f 2 26.10.2004 19:25 10 1 71 f 7 27.10.2004 19:15 10 2 74 f 7 29.10.2004 06:20 10 4 80 f 7 30.10.2004 05:31 10 16 78 f - = no data available m = male f = female X =animal escaped before it could be marked Recapture Appendix 65 Table 6: Trapping data from the fence-line study species number date 1 10.8.2004 G. paeba 1 11.8.2004 G. paeba 1 10.8.2004 A. namaquensis 1 11.8.2004 A. namaquensis 1 14.8.2004 A. namaquensis 2 10.8.2004 A. namaquensis 2 13.8.2004 A. namaquensis 2 13.8.2004 A. namaquensis 2 13.8.2004 A. namaquensis 2 14.8.2004 A. namaquensis 3 10.8.2004 A. namaquensis 3 13.8.2004 A. namaquensis 3 14.8.2004 A. namaquensis 11.8.2004 A. namaquensis X 2 10.8.2004 G. paeba 2 14.8.2004 G. paeba 429 13.8.2004 R. pumilio 433 13.8.2004 R. pumilio 91 13.8.2004 R. pumilio 91 14.8.2004 R. pumilio 141 13.8.2004 R. pumilio time area station weight (g) sex 19:56 Farm 26 24 m 19:54 Farm 26 25 m 21:22 Farm 18 50 f 21:15 Farm 19 49 08:00 Farm 19 21:22 Farm 19 37 06:50 Farm 19 37 f 09:30 Farm 19 36 f 11:20 Farm 19 06:20 Farm 19 40 f 21:35 Farm 21 46 f 06:55 Farm 21 50 f 06:31 Farm 20 50 f 19:18 Farm 21 52 21:05 Goegap 19 27 f 06:47 Goegap 13 30 f 09:20 Goegap 15 62 m 09:25 Goegap 23 50 m 11:00 Goegap 13 57 m 09:40 Goegap 13 57 m 11:05 Goegap 17 60 m - = no data available m = male f = female X =animal escaped before it could be marked Recapture Acknowledgements 66 9. Acknowledgements A lot of people made their contribution during this diploma thesis. First, I want to thank Prof. Norbert Sachser, who gave me the possibility to make this external diploma thesis and had the trust to send me half around the world in order to do it. Most of all I have to thank my supervisor Dr. Carsten Schradin. He showed a really exceptional engagement for my diploma thesis and me and even lent me his beloved Landrover to collect my data. The data collection would not have been possible without the help of my field assistants. My special thanks go to Philipp Widmann and Annette Wiedon for bearing my bad mood during many trapping nights and the effective help with dragging traps and mountain climbing. For further field assistance I thank Eva Krause, Madeleine Scriba, Melanie Schubert, Carola Schneider and Brigitte Britz. The whole team from the research station in Goegap Nature Reserve owes my great thanks for a lot of social support and motivation during difficult times. I got a lot of useful tips and lessons in distinguishing E. rupestris from E. edwardii from Galen Rathbun and therefore also send my thanks to California. For the warm reception back in Münster and a lot of advises I thank the whole team of the Department of Behavioural Biology. Most support came from Oliver Ambreé, who spent a great deal of time helping me to solve all my little problems. For the correction of my English I thank Prof. Dr. Michael Hennessy. For their patience and competent help for an ignorant zoologist, who tried to analyse soil samples, I thank Dr. Nicole Armbrüster and Hildegard Schwitte. For providing a scale and managing to bring it to South Africa I thank Prof. Dr. von Willert. I also have to thank Jens Mecklenborg for providing his laptop and bolstering me, along with many other friends and family members. Especially I want to mention my flatmate Jörg Holle, who had to deal with an interim tenant for six month and was still so generous to provide me an extra desk. I also thank Ruben Böhmer for constantly telling me that everything can be managed. I would like to thank the Northern Cape Department of Agriculture, Land Reform, and Environment for their permission to conduct my studies in Goegap Nature Reserve, and the staff of Goegap for their assistance. For financial support I thank the University of the Witwatersrand, Johannesburg and especially Prof. N. Pillay the Frauenförderung of the University of Münster and my parents, who supported me during my whole course of studies. Erklärung Hiermit versichere ich, die vorliegende Arbeit selbstständig verfasst und keine anderen als die angegebenen Quellen und Hilfsmittel verwandt zu haben. Münster, den 30.04.2005 . Christina Keller