i. calorimetry
advertisement

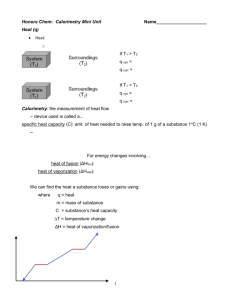
CALORIMETRY I. CALORIMETRY If the process (e.g. chemical reaction, phase conversion) requires heat to proceed, it is said to be endothermic. For endothermic process, q > 0. If the process (e.g. chemical reaction, phase conversion) evolves heat, it is said to be exothermic. For exothermic process, q < 0. Letter "q" denotes the heat flow between surroundings and the system. We often need to know the magnitude and direction of the heat flow for a process. The method by which the heat flow is measure is termed calorimetry. If you would like to measure the heat flow in a process, you must have an isolated vessel called a calorimeter. The most commonly used calorimeters base on so called Dewar’s vessel. However one can use as simple systems as e.g. two Styrofoam coffee cups placed inside the other and covered with a lid. In all our experiments the calorimeter will be filled with water the temperature of which will be measured. The calorimeter and water are called surroundings and the substance/s undergoing an investigated process is/are called system. The specific heat (C) of a substance is that amount of heat which is required to increase the temperature of one gram of a substance by one Kelvin (or one degree Celsius). The heat capacity of the calorimeter (K), sometimes denoted as calorimeter constant, is that amount of heat which is required to increase the temperature of calorimeter by one Kelvin (or one degree Celsius). Note the difference between specific heat and heat capacity. We cannot measure heat directly. Instead, we measure the change in temperature of the water. The equation which relates heat flow, temperature change and specific heat is: Where m is the mass of the substance, C is the specific heat, and ΔT is the change in temperature (Tfinal-Tinitial). This equation can be used to calculate the amount of heat gained or lost by water on proceeding the investigated process. For endothermic process, q > 0: Lodz University of Technology, Faculty of Chemistry, Institute of Applied Radiation Chemistry, Laboratory of Chemistry CALORIMETRY Because the calorimeter is a closed system, all the heat gained by the changing system has to be lost by water. Therefore we can write the following equation: For exothermic process, q < 0: Because the calorimeter is a closed system, all the heat evolved by the changing system has to be gained by water. Therefore we can write the following equation: These equations imply that the heat lost/gained by the system is equal and opposite to the heat gained/lost by the water. One should interpret the negative sign to mean "opposite to". If we take into account that the real calorimeter is a setup consisting of dewar vessel, water, stirrer, termometer, the product mwCw in equations (3) and (4) should be replaced with the heat volume of calorimeter (K), i.e.: Equations (4a) and (5a) can be used to calculate heat lost/gained by the system (q process) when we know the values of K and ΔTw or to calculate K, when we know the values of q process and ΔTw. Lodz University of Technology, Faculty of Chemistry, Institute of Applied Radiation Chemistry, Laboratory of Chemistry CALORIMETRY I.A. HEAT OF MELTING OF ICE Purpose: to learn the principles of calorimetry measurements, to determine the heat of melting of ice Procedure: 1. Weigh the empty Dewar vessel. 2. Fill the Dewar vessel with 50.0 mL of ~30 °C water and weigh the vessel. Record the mass. 2. Measure and record the initial temperature of the water. 3. Add 2-3 ice cubes to the water. Stir gently with the temperature probe. Before the ice is completely melted, add another ice cube. 4. Continue adding ice cubes in this manner until the temperature reaches a plateau. Record the final temperature. 5. Carefully pull out the remaining ice cubes, allow the water to drop back into the vessel. 6. Weigh the vessel and record the mass. Calculations: 1. Find the mass of water originally in the cup. 2. Calculate the amount of heat the water gave to the ice cubes. Use the mass of water and the change in temperature. 3. Calculate the mass of ice which melted. 4. The amount of heat released by the water (and calculated in step 2) is equal to the amount of heat absorbed by the ice. Knowing this and the mass of the ice which melted, calculate the heat of melting of ice in units of J/g. Recalculate it to kJ/mole of ice. 4. Find the error in %. Include in the introduction the definition of heat, the definition of heat of melting, describe the calorimetry and how it works. Give the accepted values for the heat of melting of ice, in both kJ/mole and J/g. Make sure all equations used are clearly shown in your calculations. Lodz University of Technology, Faculty of Chemistry, Institute of Applied Radiation Chemistry, Laboratory of Chemistry CALORIMETRY I.B. HEAT OF SOLUTION OF A SOLID Purpose: to learn the principles of calorimetric measurements, to observe the dependence of the heat of solution on the concentration of the solution formed. The process of solid dissolution in water is always associated with energy change (heat). The dissolving process itself is a two-step process. The first step, that of breaking down the solid crystal, is endothermic. The second step, that of hydrating the individual particles released into the solvent, is exothermic. For an ionic compound, ionic bonds are broken while ion-dipole interactions appear and the ionic compound dissociates into ions, negative and positive ones. Ion-dipole interactions appear when the slightly positive hydrogen atoms of water molecule are attracted to the negative ions, and the slightly negative oxygen atoms of the water molecule are attracted to the positive ions. We talk about the hydration of ions. The overall heat of solution depends on the relative amounts of energy involved in the two individual steps. Hydration of ions involves a complex redirection of forces of attraction and repulsion. Before the solution forms, water molecules are attracted only to each other; and positive and negative ions have only each other in the crystal to be attracted to. In the solution, the ions have water molecules to take the places of their oppositely charged counterparts; and water molecules find ions more attractive then even other water molecules. In this experiment, you will determine the heats of solution for the dissolving of the same amount of a salt (e.g. ammonium nitrate, sodium acetate, sodium thiosulphate) in different amounts of water using a Dewar vessel as a calorimeter. Procedure: 1. Accurately weigh a sample of solid salt of approximately 5 grams. 2. Add exactly 500 mL of water to the Dewar vessel. 3. Stir the water briefly with the stirrer and start recording the temperature every 30 s. 4. Dissolve the solid in the water, stirring continuously, and continue recording the temperature. 5. Rinse out the vessel, dry it thoroughly, and repeat the experiment twice adding 250 mL of water. Lodz University of Technology, Faculty of Chemistry, Institute of Applied Radiation Chemistry, Laboratory of Chemistry CALORIMETRY Calculations: 1. Calculate the following for each part of the experiment. a) the temperature change of the water. (Hint: make a plot of temperature versus time of observation.) b) the quantity of heat gained (or given off) by the water during the dissolving, K=2,5 kJ/deg. c) the number of moles of salt used. e) the molar heat of solution, i.e. the quantity of heat involved per mole of salt dissolved. 2. Find the accepted values for the molar heats of solution for these solids in a chemistry handbook, and calculate the percentage error of your experimental values. Observe whether the numbers given in a handbook concern the molar ratio of salt and water. Questions: 1. Write an equation for the dissolving process for each salt adding the heat term to each equation. 2. Describe each step of the two-step process of dissolution using both words and a diagram. Designate each step as endothermic or exothermic. 3. Which of these individual steps shows the higher absolute value? Lodz University of Technology, Faculty of Chemistry, Institute of Applied Radiation Chemistry, Laboratory of Chemistry CALORIMETRY I.C. HEAT OF NEUTRALIZATION REACTION The neutralization reaction The reaction of sulfuric acid with sodium base can be written as follows: or in an ionic form: It appears that the reaction proceeds between hydrogen cations and hydroxyl anions and results in the formation of water. Definition: the neutralization reaction of strong acid with strong base is the reaction of hydrated hydrogen cations with hydrated hydroxyl anions resulting in the formation of water: Heat of this reaction does not depend on the type of acid or base and equals -56.61 kJ per mole of water formed. Procedure: 1. Fill a glass ampoule with ca. 3 g of sulfuric acid (you should know the exact mass of acid). Ask TA for assistance. 2. Calculate what volume of NaOH water solution (20% w/w) is needed to neutralize the amount of acid you have in an ampoule. Ask TA for the acceptance of your calculations. 3. Place the calculated volume (increased by 30%) of NaOH solution into an Dewar vessel and add water so that the final volume of the obtained solution would be exactly 500 mL. 4. Mount the ampoule containing sulfuric acid in the holder provided with the calorimter setup. Connect stirrer to the electric engine. Place properly the temperature probe in Dewar vessel. 5. Stir the water briefly and start recording the temperature every 30 s. 6. Allow sulfuric acid to dissolve in the solution by breaking smoothly the glass ampoule. Do not stop stirring and continue recording the temperature until its value reaches plateau. Lodz University of Technology, Faculty of Chemistry, Institute of Applied Radiation Chemistry, Laboratory of Chemistry CALORIMETRY 7. Dismount carefully the calorimeter setup. Rinse Dewar vessel with water. Rinse the broken ampoule with water and give it back to the TA. Calculations: 1. Make a plot of temperature versus time and read out the temperature change (increase). 2. Calculate the heat flow observed on dissolving sulfuric acid in the water solution of sodium base. 3. Note that the observed heat flow results from the two processe taking place simultaneousely in an Dewar vessel. They are: (i) dissolution od sulfuric acid in water and (ii) neutralization reaction: therefore 4. Find the heat of sulfuric acid solution in water in a chemical handbook. Calculate the observed heat of neutralization taking the heat capacity of the calorimetric setup a 2.5kJ/deg. Hint: heat values shown in tables refer to one mole of sulfuric acid, while you have used much lower amount. 5. Calculate the heat of neutralization for one mole of water formed. Hint: From one mole of fully neutralized sulfuric acid two moles of water are formed. 6. Compare the obtained molar value of the heat of neutralization with accepted value. Comment possible differences. Lodz University of Technology, Faculty of Chemistry, Institute of Applied Radiation Chemistry, Laboratory of Chemistry CALORIMETRY I.D. DETERMINING THE HEAT CAPACITY OF CALORIMETER Purpose: to learn the principles of calorimetry measurements, to determine the heat capacity of calorimeter, i.e to calibrate the calorimeter Procedure: 1. Fill a glass ampoule with ca. 2,5 - 3 g of sulfuric acid (you should know the exact mass of acid). Ask TA for assistance. 2. Add exactly 500 ml of water to the Dewar vessel. 3. Place the ampoule, stirrer and thermometer probe on their places. 4. Stir the water briefly with the stirrer and start recording the temperature every 30 s. 5. Allow acid to dissolve in water by gentle breaking the glass ampoule. Continue recording temperature until its value reaches plateau. 6. Dismount carefully the calorimeter setup. Rinse Dewar vessel with water. Rinse the broken ampoule with water and give it back to the TA. 7. Repeat steps 2 – 6 taking 5 -6 g of sulfuric acid. Calculations: 1. Make a plot of temperature versus time and read out the temperature change (increase). 2. Find the heat of sulfuric acid solution in water in a chemical handbook and calculate the heat loss (qprocess) during the process of acid dissolution in water. Hint: heat values shown in tables refer to one mole of sulfuric acid, while you have used much lower amount 3. Calculate the heat capacity of the calorimeter. 4. Compare the result of the two experiments. Does the heat capacity of the calorimeter depend on the amount of acid taken? Haw could you easily change the heat capacity of your calorimeter? Lodz University of Technology, Faculty of Chemistry, Institute of Applied Radiation Chemistry, Laboratory of Chemistry