O HO OOO NH2 O - Mercyhurst University
advertisement
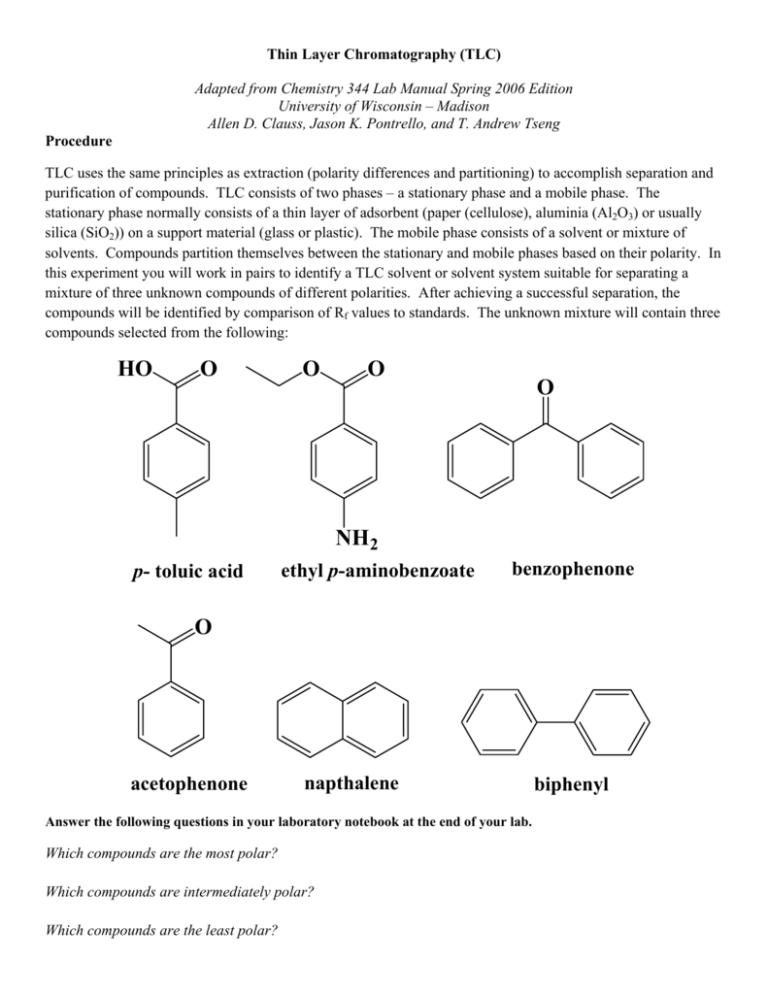
Thin Layer Chromatography (TLC) Adapted from Chemistry 344 Lab Manual Spring 2006 Edition University of Wisconsin – Madison Allen D. Clauss, Jason K. Pontrello, and T. Andrew Tseng Procedure TLC uses the same principles as extraction (polarity differences and partitioning) to accomplish separation and purification of compounds. TLC consists of two phases – a stationary phase and a mobile phase. The stationary phase normally consists of a thin layer of adsorbent (paper (cellulose), aluminia (Al2O3) or usually silica (SiO2)) on a support material (glass or plastic). The mobile phase consists of a solvent or mixture of solvents. Compounds partition themselves between the stationary and mobile phases based on their polarity. In this experiment you will work in pairs to identify a TLC solvent or solvent system suitable for separating a mixture of three unknown compounds of different polarities. After achieving a successful separation, the compounds will be identified by comparison of Rf values to standards. The unknown mixture will contain three compounds selected from the following: HO O O O O NH2 p- toluic acid ethyl p-aminobenzoate benzophenone O acetophenone napthalene Answer the following questions in your laboratory notebook at the end of your lab. Which compounds are the most polar? Which compounds are intermediately polar? Which compounds are the least polar? biphenyl TLC Technique (see Figure 1) Application of the Sample The sample to be separated is generally applied as a small spot (1 to 2 mm diameter) of solution about 1 cm from the bottom of the TLC plate. The addition may be made with a “spotter” – a small diameter glass tube that pulls the sample up by capillary action. As small a sample as possible should be used, so only dot the TLC plate with your sample once. Do not disturb the silica gel absorbent when you make the spot (tear it or gouge a hole in it). This will result in uneven flow of solvent. Several samples can be analyzed on one plate simultaneously by spotting them on a horizontal line at the bottom of the plate, leaving about 0.5 cm between spots. The starting position of the spots can be indicated by a small pencil mark near the edge of the plate. Do not use pen! Development A beaker with a watch glass over it can be used for development of the TLC plates. The developing solvent is poured into a beaker to a depth of about 0.5 cm. The spotted plate is then placed in the chamber, spotted end down. The solvent level must be below the spots. The solvent will slowly rise in the adsorbent (silica) by capillary action. In order to get reproducible results, the atmosphere in the development chamber must be saturated with the solvent. This can be accomplished by placing a filter paper “wick” liner around the inside of the jar and sloshing the solvent around in the contained before any plates have been added. The atmosphere in the chamber is then kept saturated by keeping the container closed at all times except for the brief moment during which a plate is added or removed. Visualization When the solvent front has moved to within about 1 cm of the top of the adsorbent, the plate should be removed from the developing chamber, the position of the solvent front marked with pencil, and the solvent allowed to evaporate in the hood. If the components of the sample are colored, they can be observed directly. If not, they can be visualized by using adsorbant containing a fluorescent additive and shining ultraviolet light on the plate. Organic compounds generally show up under UV light as dark purple spots against a fluorescent green background. If the organic compounds are not UV sensitive, a stain must be applied to the plate to visualize the spots. Circle where the spots show up on your TLC plate in pencil. Using this information you can calculate the Rf value for each of your compounds. Rf valued are calculated by dividing the distance the substance traveled divided by the distance the solvent front traveled. The Rf value should be a number less than one and is a characteristic of that compound. The Retention factor (Rf) is defined as; Rf = distance traveled by substance distance traveled by solvent front Figure 1. Representation of a TLC plate. Choosing a Solvent System The process of moving compounds up the TLC plate with solvent is called elution. The less polar a solvent is the less eluting power it has. The more polar a solvent is the greater eluting power it has. More polar compounds tend to stick to the polar adsorbent and move a smaller distance on the TLC plate (spot A). Less polar compounds spend more time in the mobile phase, which is generally less polar then the adsorbent, and move further up the TLC plate (spot C). Below is a list of the common solvents and functional groups and their relative polarities. You will have access to hexanes, toluene, ethyl acetate, and acetone. Ethanol and water are generally much too strong of eluters to use for a successful separation. We have consciously omitted dichloromethane in order to “green” our laboratory procedure as halogenated solvents are particularly harmful to the environment. Use this information as a guide when deciding on a solvent system. Solvents for Chromatography Least eluting power Hexanes Toluene Dichloromethane Ethyl Acetate Acetone Ethanol Greatest eluting power Water Adsorptivity of Organic Compounds by Functional Group Least Strongly Adsorbed Alkanes Alkenes Aromatics Ethers Esters Aldehydes and Ketones Alcohols Most Strongly Absorbed Carboxylic Acids or Amines Separation of Unknowns You will work in pairs to devise a strategy for testing solvents and identifying one or more solvents that can be used to separate the unknown mixture. Consider the range of polarities represented by all of the possible compounds on the previous page when determining which solvent to use. Group members will simultaneously test different solvent systems and compare the results to determine the best solvent(s) to achieve separation of the mixture. It may be necessary for you to use a binary solvent system (consisting of a mixture of two solvents) to separate all three compounds in your mixture. Sketch the TLC plates in your lab notebook (original spot, where each unknown shows up, and the solvent front) and label the solvent system used beneath it. Be sure to note if any spots were only visible under UV light. Record Rf values of the compounds in the different solvent systems you use. Report the Rf values for your successful separation of the compounds in your data summary at the end of the lab. Identifying the Unknown Compounds The next step is to work with your partner to identify the three compounds in your unknown mixture by comparison to standards of the pure compounds. Each group should run the TLC of the standards using the optimal solvent condition determined in the previous section. A group member can run a TLC separation of the unknown mixture along with a few of the standards spotted on the same plate. By comparing the Rf values of the standards to those of the compounds in the unknown mixture, the identity of the unknown compounds is determined. If there is more than on standard with a Rf value close to one of your unknown compounds it may be necessary to run one or more additional TLC experiments in which the standards of similar Rf are carefully spotted and run side by side with the unknown mixture on the same plate.








